Journal of Cancer Research and Immuno-Oncology
Open Access
ISSN: 2684-1266
ISSN: 2684-1266
Research Article - (2022)Volume 8, Issue 4
The humoral response of the COVID-19 vaccine varies from person to person. It largely depends on prior SARS- CoV-2 infection, obtaining an adequate immune response, and leaving a trace of changing antibody concentration over time. We retrospectively analyzed five clinical cases from selected patients and employees of the oncology hospital. All mild COVID-19 convalescents received the BNT162b2-Comirnaty mRNA vaccine three or four times. The levels of SARS-CoV-2 IgM- and IgG-specific antibodies, as well as S-RBD antibodies, were analyzed for two years. The concentration of antibodies was assessed in the laboratory using the chemiluminescent immunoassay CLIA, MAGLUMI.
Results: (1) Active autoimmune disease stabilized the level of IgG-specific antibodies after systemic mRNA vaccination for at least six months. (2) Post-vaccination IgG and S-RBD levels decreased when vaccination was performed within three months of onset. (3) The booster dose administered only increased the S-RBD antibody levels. Declining IgG-specific antibodies were observed. (4) The S-RBD IgG levels were not correlated with the SARS-CoV-2 IgG levels in the vaccinated convalescents. (5) Subsequent reinfection with SARS-CoV-2 after vaccination three times released a more significant specific antibody response. Based on the collected data, we suggest that monitoring S-RBD antibodies is sensitive but not equivalent to a specific humoral response for SARS-CoV-2 IgG. We suggested that administering at least three doses of the mRNA vaccine should serve as the basis for immunization. The three- month interval may be the best alternative to an immunization schedule for non-immunocompromised people.
COVID-19; BNT162b2; Vaccination; S-RBD; SARS-CoV-2; Seroconversion
The common SARS-CoV-2 virus has infected approximately 600 million people across 228 countries worldwide, leaving behind natural immunity [1]. Acute COVID-19 infection can cause a cytokine storm that leads to Acute Respiratory Failure (ARDS) and death [2], while severe inflammatory disease can result in chronic Polymyositis Syndrome in Children (PIMS) [3]. Almost half (44.8%) of symptomatic infections in children and adults manifest as varied symptoms related to chronic infection, in the form of long-COVID/post-COVID clinical syndrome, cognitive and physical deficits, pulmonary fibrosis, myocarditis, or neurological deficits [4,5]. The findings so far show that to acquire antiviral immunity to SARS-CoV-2, one does not need to come into contact with the virus and become infected [6]. Being around immunized people can provide a cellular and humoral response. To what extent is it active and provides self-immunity? How to check if we have acquired the appropriate anti-virus response, or only had contact with it? When should a booster be given? The situation applies to everyone, including the healthy, those sick with a viral disease in any stage, those with impaired immunity, and young people. The data we collected show that it is necessary to measure the concentration of IgG-specific antibodies after the onset of SARS-CoV-2 and to measure the concentration of post-vaccine IgG (anti-S, anti-RBD, and S-RBD) [6]. IgM and IgG antibody levels may indicate early- or late-phase infection [7]. Administration of antibody response acquisition during the early phase has only demonstrated therapeutic implications for symptomatic COVID-19 cases [8], and vaccination of people with a history of SARS-CoV-2 without knowledge of their humoral response is considered safe [9]. In our hospital, most medical staff and patients underwent vaccinations without knowing that they had acquired a humoral response beforehand [10].
The decline in humoral immunity over time following coronavirus infection, including SARS-CoV-2, is typical in mild cases [11-16]. Then, do all infections require antibody monitoring? [17] Found that after administering a second dose of the vaccine (complete vaccination) in cancer patients, the individual humoral anti-S antibody response level could be determined after three to four weeks. It has been proven that the level of S-RBD antibodies is correlated with protection against symptomatic SARS-CoV-2 infection [18,19], and not asymptomatic infection [20]. Thus, after the third dose and subsequent boosters, does the level of only anti-S antibodies determine immunity? In a selected population of healthy people, the level of the humoral response can last up to 13 months [19]. During our study, the Delta strain of SARSCoV- 2 accounted for over 95% of infections in Poland. At that time, rapid neutralization by current vaccines of the new Delta virus [20, 21] was reported after complete inoculation with BNT162b2. We asked whether, during the next wave of infections in Europe with the different Omicron virus types, the administration of a booster in healthy people would stimulate the immune system again [22,23], or if it would only cause an increase in the S-RBD in response to the vaccine? The description of five different courses of SARS-CoV-2 infection until the administration of subsequent doses of the Comirnaty vaccine shows that a specific immune response expires approximately one year after the onset of the disease and is not induced after subsequent artificial immunization [10]. We call this effect vanishing antibodies. Another illness with a new variant of the SARS-CoV-2 virus can stimulate a new response to a different level [24-26]. We call this a new set-up. In other data, currently unpublished, article focused on a year-long case observation of the COVID-19 humoral response, we saw that an early double infection (without or after vaccination), rather than a third booster, raises antibodies to a higher "protective" IgG level.
Before vaccines were introduced in 2021, the natural immunity in the SARS-CoV-2 pandemic was investigated by analyzing antibody concentrations. Antibody measurements were analyzed routinely for oncology patients and hospital staff for medical purposes during the COVID-19 pandemic. Retrospectively, we decided to follow five previously selected people for approximately 24 months—with most measurements taken monthly (timeline) (Supplementary Table). The complete characteristics of the antibody collection in five subjects (Case #1,2,3,4,5) in months (samples timeline). Event: SARS-CoV-2 infection time (positive PCR or antigen test); Com1, Com2, Com3, Com 4 – vaccination time (Comirnaty).
Three patients (#2, #4, #5) and two hospital staff persons (#1,#3) were chosen. Case #2 was chosen because of their rapid response to SARS-CoV-2 infection (hyper-responder with SARS-CoV-2 IgG >2000 AU/mL in less than 14 days), providing an excellent case of early seroconversion relevant to acute viral infection. The other two persons were selected as the controls, one being COVID-19 convalescent (#3) and one lacking a SARS-CoV-2 history (#1). The other two patients passed COVID-19 twice with mild (#4) and severe (#5) respiratory problems. Case #3 stayed at the hospital and was cured against COVID-19 using remdesivir (Veklury) for five days. Case #1 patient was chosen because of a lack of a history of COVID-19, but analyses of his seroconversion proved past SARS-CoV-2 infection (IgG cut-off >0.2 AU/mL). Case #4 stayed and cured COVID-19 at home. Case #5 remained at the hospital because of bilateral pneumonia and was given steroids and early plasma antibodies. All five were vaccinated with BNT162b2 mRNA (Comirnaty) according to the manufacturer's schedules and took booster vaccines at different times (gray arrows in figures; Com1, Com2, Com3, Com4).
Characteristics of the people
Case #1: Thirty-four-year-old Caucasian male, no chronic diseases, non-smoker, undergoing hypertension treatment.
Case #2: Sixty-eight-year-old Caucasian women, with chronic diseases (rheumatoid arthritis and degeneration of the spine), non-smoker, hypertension treatment, taking NSAIDs and treated with NSAIDs for COVID-19 infections at home.
Case #3: Forty-nine-year-old Caucasian male, hospitalized early for COVID-19 because of headaches and a cough, shortness of breath (saturation 88%), with five days of remdesivir (Veklury) treatment.
Case #4: Fifty-nine-year-old Caucasian woman, non-hospitalized for COVID-19. First time SARS-CoV-2 infection with flu, second with mild gastrointestinal problems.
Case #5: Sixty-eight-year-old Caucasian male postponed the urgent urology operation because of a mild second SARS-CoV-2 infection in 2021. Now is under prostate cancer hormonotherapy. Chest CTs for Cases #2, #3, and #5 showed no post-COVID changes three months after treatment
Materials: Our study materials were blood specimens taken through venipuncture sampling. The concentration of antibodies was evaluated four hours after blood collection. If an immediate assessment was impossible, the serum was collected and stored at –80˚C. Due to the fact that most of the data (2021) are in AU/mL, the results of measurements in BAU/mL in 2022 were converted into AU/mL.
Methods: The antibody concentrations were detected by the chemiluminescent immunoassay CLIA (MAGLUMI, Snibe Diagnostic, Shenzhen, China). Per the manufacturer's protocol, results greater or equal to 1.0 AU/mL of SARS-CoV-2 IgG, IgM, and S-RBD were considered reactive and positive. The maximum limits of the antibody measurements were assumed: 2000 AU/mL of S-RBD (initially, it was 100 AU/mL), with the upper limit of the SARS-CoV-2 antibody measurements being 2000 AU/mL. According to our earlier observations in this population, the cut-off value for SARS-CoV-2 IgG should be >0.2 AU/mL for a positive test result. Antibody tests were ordered by a hospital doctor at different times, from vaccination to illness. The results are presented in supplementary Table1 depending on the time of the test (timeline). The patients provided their written consent for performing humoral immunity tests and for participating in this study. The Bioethics Commission of the Medical University provided consent for our research.
The immunization (infection ad vaccination) results for each person are shown as a timeline presented in five diagrams for each (Figures 1,2,3,4 and 5). The diagrams are presented as follows:
(a) SARS-CoV-2 IgM with IgG;
(b) SARS-CoV-2 IgG with S-RBD IgG;
(a) and (b) or (b) and (c) is a part of the diagram (a) or (b), respectively, to explain the dynamic changes of humoral response;
(c) All three antibody concentrations are time-related in the logarithmic chart.
Figures 1–5 presents the humoral response of each person after infection and vaccination in time. The legend in the figures shows arrows indicating systemic vaccinations (Com1, Com2, Com3, Com4) and the symbol of the virus pointing to SARS infections.
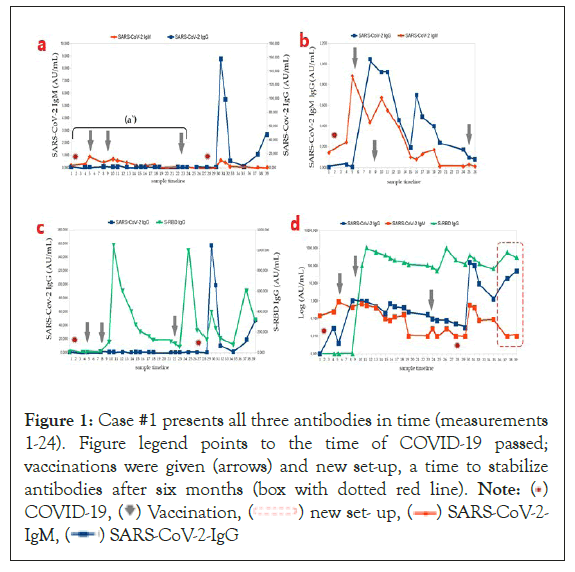
Figure 1: Case #1 presents all three antibodies in time (measurements
1-24). Figure legend points to the time of COVID-19 passed;
vaccinations were given (arrows) and new set-up, a time to stabilize
antibodies after six months (box with dotted red line). Note: ( )
COVID-19, (
)
COVID-19, ( ) Vaccination, (
) Vaccination, ( ) new set- up, (
) new set- up, ( ) SARS-CoV-2-
IgM, (
) SARS-CoV-2-
IgM, ( ) SARS-CoV-2-IgG
) SARS-CoV-2-IgG
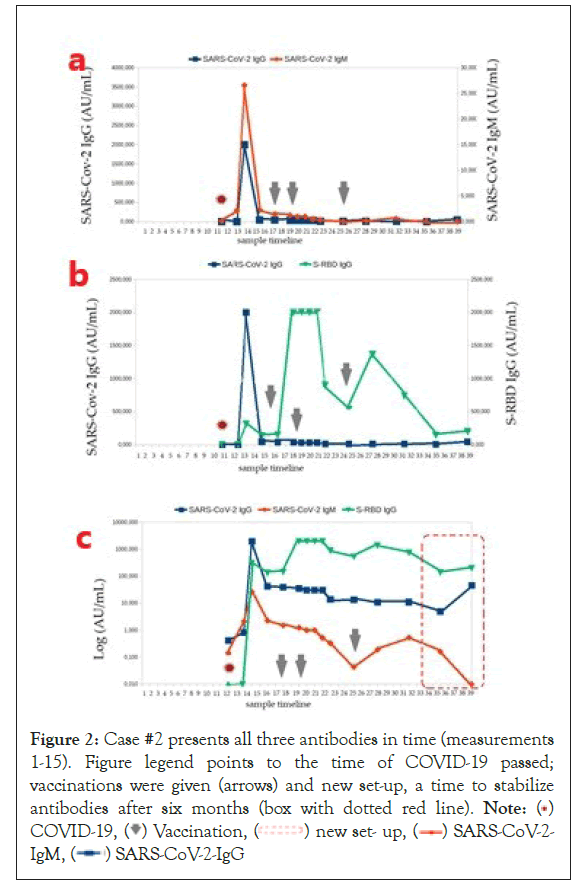
Figure 2: Case #2 presents all three antibodies in time (measurements
1-15). Figure legend points to the time of COVID-19 passed;
vaccinations were given (arrows) and new set-up, a time to stabilize
antibodies after six months (box with dotted red line). Note: ( )
COVID-19, (
)
COVID-19, ( ) Vaccination, (
) Vaccination, ( ) new set- up, (
) new set- up, ( ) SARS-CoV-2-
IgM, (
) SARS-CoV-2-
IgM, ( ) SARS-CoV-2-IgG
) SARS-CoV-2-IgG
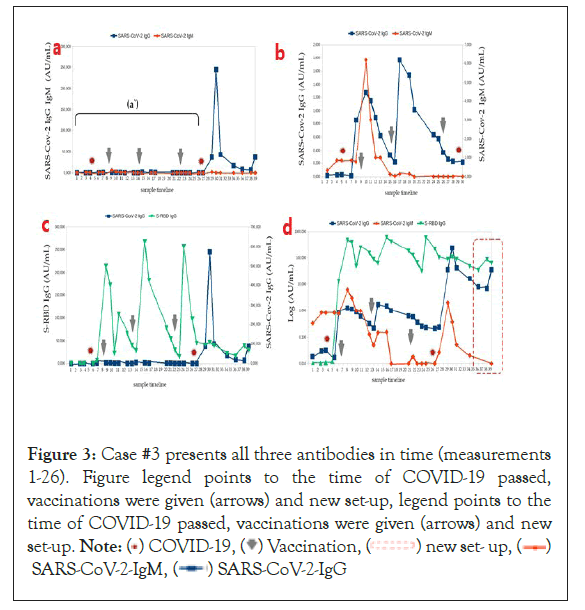
Figure 3: Case #3 presents all three antibodies in time (measurements
1-26). Figure legend points to the time of COVID-19 passed,
vaccinations were given (arrows) and new set-up, legend points to the
time of COVID-19 passed, vaccinations were given (arrows) and new
set-up. Note: ( ) COVID-19, (
) COVID-19, ( ) Vaccination, (
) Vaccination, ( ) new set- up, (
) new set- up, ( )
SARS-CoV-2-IgM, (
)
SARS-CoV-2-IgM, ( ) SARS-CoV-2-IgG
) SARS-CoV-2-IgG
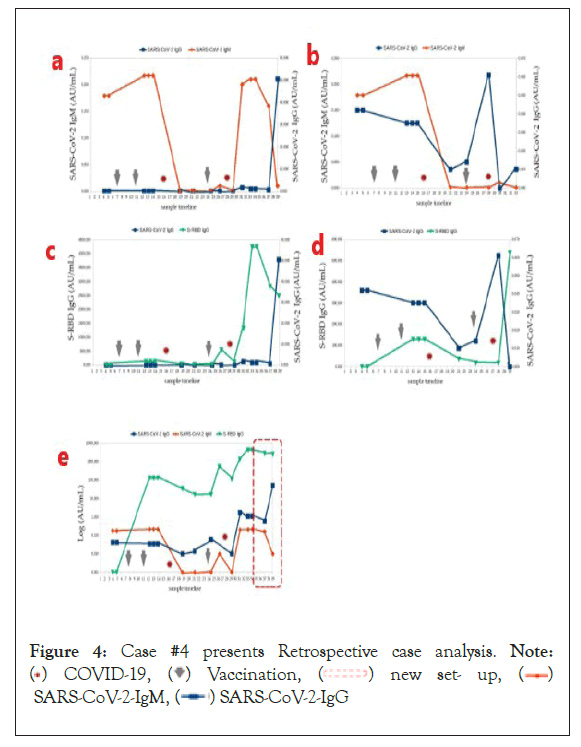
Figure 4: Case #4 presents Retrospective case analysis. Note:
( ) COVID-19, (
) COVID-19, (  ) Vaccination, (
) Vaccination, ( ) new set- up, (
) new set- up, ( )
SARS-CoV-2-IgM, (
)
SARS-CoV-2-IgM, ( ) SARS-CoV-2-IgG
) SARS-CoV-2-IgG
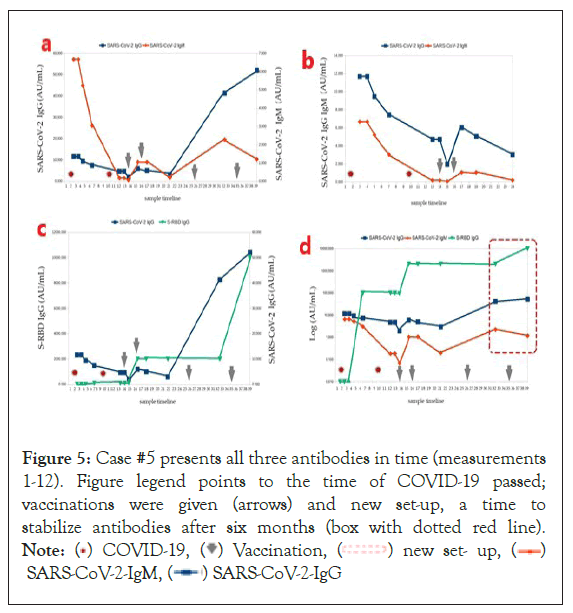
Figure 5: Case #5 presents all three antibodies in time (measurements
1-12). Figure legend points to the time of COVID-19 passed;
vaccinations were given (arrows) and new set-up, a time to
stabilize antibodies after six months (box with dotted red line).
Note: ( ) COVID-19, (
) COVID-19, ( ) Vaccination, (
) Vaccination, ( ) new set- up, (
) new set- up, ( )
SARS-CoV-2-IgM, (
)
SARS-CoV-2-IgM, ( ) SARS-CoV-2-IgG
) SARS-CoV-2-IgG
Case #1: (a) All IgG and IgM antibodies (measurements 1–24). A retrospective case study showed that the person had had SARS-CoV-2 infection in October 2020 without clinical symptoms, so we present an additional diagram with only 1–20 measurements separately to point out this phenomenon (Figure 1). There is a typical seroconversion, seen as an increase in IgM and IgG, assumed as positive results (cutoffs: IgM >1.0 AU/mL and IgG >0.2 AU/mL). The results should be considered clinically asymptomatic of previous SARS-CoV-2 infection. As hospital staff, he was routinely vaccinated in January 2021 with two doses of BNT162b2 (Comirnaty) on a schedule over 28 days (see arrows). (b) Post-vaccination effects are seen as S-RBD gains and triple antibody re-synthesis in SARS-CoV-2 infection and vaccination (b) The three peaks of IgM and IgG mean three seroconversions in a row). (c) Logarithmic representation of the three antibody concentrations. Notable here is a conversion to higher SARS-CoV-2 IgG concentrations than baseline before SARS-CoV-2 infection at the beginning of the diagram. A booster was given eight months after the second dose of Comirnaty (see arrow) due to another wave of illness (autumn) for medical personnel (only increased S-RBD). On the contrary, at that time, the second vaccination did not work (b) only increased S-RBD after the booster, but together with COVID-19 (raised S-RBD with SARS-CoV-2 IgG). This phenomenon is called enhanced humoral response without classical seroconversion (fast release IgGspecific antibodies). (c) After a few months, he passed SARS-CoV-2 asymptomatically, which provided a new set-up of humoral response (in the red frame).
Case #2: Figure 2 Perspective case analysis; the person, had symptomatic COVID-19 infection in March 2021, with a sore throat and muscles, a fever, and the chills. (a) Antibody collection for assays was routinely scheduled (considered a hyper-responder) as a hospital-monitored oncology patient, vaccinated in July 2021 with two doses of BNT162b2 (Comirnaty) on a schedule over 28 days (see arrows). A booster was given after half a year delay, as an auto-immunologic person. (c) A logarithmic representation of the three antibody concentrations. (b) Post-vaccination effects, shown as an increase in S-RBD extended to six months and no re-synthesis of antibodies in the vaccine prompt. (b,c) No fluctuations in the IgM and IgG concentrations. The conversion to higher IgG concentrations before SARS-CoV-2 infection and the exceptional smooth/stable course of the immune antibody curves are noteworthy. (c) A new set-up of antibodies can be seen in the red frame.
Case #3: (a) Retrospective case analysis. The person had symptomatic COVID-19 infection in December 2020, including a sore throat, a cough, a fever, and dyspnea. Routinely, antibodies were collected for assay (Figure 3); during inpatient treatment, remdesivir (Veklury) was administered for five days while being monitored by medical personnel in the hospital, and vaccinations occurred in January and in May 2021 with two doses of BNT162b2 (Comirnaty) because this patient underwent an urgent spine operation in March. A booster was given five months after the second dose of Comirnaty because of lost antibodies. SARS-CoV-2 IgG of 0.26 AU/mL and S-RBD IgG of 32.40 AU/mL (see arrows). (b) Here, we can see the post-vaccination effects, shown as an increase in both S-RBD and SARS-CoV-2 IgG extended to over three months, as well as a decrease in IgG antibodies in the prompt for SARS-CoV-2 (IgG=0.22 AU/mL and IgM=0.05 AU/mL) and vaccination (S-RBD IgG=63.56 AU/mL) after five months.
Case #4: Figure 4 presents all three antibodies in time (measurements 1-15). Figure legend points to the time of COVID-19 passed; vaccinations were given (arrows) and new set-up, a time to stabilize antibodies after six months (box with dotted red line).
Case #5: Figure 5 presents all three antibodies in time (measurements 1-12). Figure legend points to the time of COVID-19 passed, vaccinations were given (arrows) and new set-up, a time to stabilize antibodies after six months (box with dotted red line).
Due to the significant decrease in immunity, the antibodies reduction, and the patient’s clinical condition, a third dose of the vaccine was used (Figure 3). After a few months, he passed into mild symptomatic COVID-19, cured after five days by molnupiravir (Lagevrio), which provided a new set-up of humoral responses (Figure 3) (in the red frame).
A logarithmic representation of all three antibody concentrations can be seen in Figures 1c, 2c, 3c, 4c and 5c. The effect of the new increasing level of IgG antibodies was obtained. Conversion to higher IgG concentrations was noticeable (after disease and after complete vaccination) initially after infection with SARS-CoV-2 and a symmetrical course of S-RBD IgG curves of immune antibodies in the time immediately after the third dose of Comirnaty. There was a difference in antibody surge after COVID-19 getting sick with the boosted vaccine (third dose) between a symptomatic patient (Figure 3b) and SARS-CoV-2 asymptomatic patient (Figure 1). The symptom convalescent patient had a higher IgG-specific level.
Following autoimmunity (Case #2), the antibody response after disease and inoculation was stable.
Significantly, it was strengthened by administering the first mRNA vaccine, respectively, after three months. The results indicate that the active autoimmune disease stabilized the level of specific IgG after systemic mRNA vaccination for at least half a year.
Looking at all cases of vaccinated convalescents, we believe a booster dose should be given later than 28 days after SARS-CoV-2 infection. Then, we can observe fluctuations in antibodies, suggesting a change in response to the subsequent immunization, depending on the patient's condition.
We expected a longer duration of neutralizing antibodies after vaccination. The level of post-vaccine IgG antibodies decreased when vaccination was performed within three months after disease onset, depending on the patient's condition. We can change this by appropriately tracking S-RBD IgG and SARS-CoV-2 IgG antibodies one month after the disease [17]. According to observations, raising IgG-specific antibodies will better occur by administering the vaccine's second (next) dose three months after the first. The administered booster dose (third dose) only increased the concentration of S-RBD antibodies. Despite the decrease in specific IgG, their level was higher than before disease and vaccination. Declining IgG-specific antibodies were observed.S-RBD IgG levels are not correlated with SARS-CoV-2 IgG levels in vaccinated convalescents [10], but they are related to symptomatic ones [16]. Surprisingly, there was no apparent spike in IgG antibody levels after the third dose of the vaccine after six months, despite a persistently elevated level. Based on the collected data, we suggest that monitoring S-RBD antibodies is sensitive, but not equivalent to a specific humoral response for SARS-CoV-2 IgG. The observations of the five presented cases show that the measurement of S-RBD correlates with specific IgG only in the period from one to three months following disease and inoculation, but not for the booster doses given in the six months after the initiation of vaccination.
Many clinical data indicate that vaccination with a third dose of Comirnaty is useful in immunocompromised people [10,11], the chronically ill [12], or people undergoing oncological treatment [13, 14]. An analysis of specific antibody concentration charts at a cut-off for SARS-CoV-2 of >0.2 AU/mL was used [10], allowing us to observe asymptomatic COVID-19 and the natural course of the disease [15]. Patterns of antibody seroconversion in response to SARS-CoV-2 infection and the BNT162b2 mRNA vaccine were apparent in the five separate cases. In the case of Case #3, it turned out to be effective. Currently, a wave of infection with the SARS-CoV-2 virus strain Omicron is going through Europe and the USA, as the BNT162b2 vaccine is effective against this strain.
The ideal past seroconversion is when IgG antibodies are formed after stimulation and are maintained for longer. For Case #1, Comirnaty vaccination needed to be postponed one more month after getting sick; for Case #2, double vaccination did not show/ change anything (excess antibodies); for Case #3, in terms of the vaccination, the third dose significantly improved the initial level of antibodies, indicating that it is safe and advisable, and similarly to Case #1, the first dose of the Comirnaty vaccine was taken too early. Analyzing a new set-up in these five cases shows that in Case #2, subsequent infection was not needed to reach a stable level of IgG antibodies [27,28].
To sum up, administration of the vaccine too early in the case of people with excess antibodies (e.g., Case #2) does not produce the expected effect, as too early an administration (Cases #1 and #3) does not allow to hit the serological window after falling ill. Following re- administration (Case #3) after five months from the administration of the first dose, based on individual indications for vaccination, the level of antibodies responds correctly.
The analysis of these five cases of antibody changes over time during the COVID-19 pandemic provides students training material and allows them to observe and control the effects of treatment—including when to administer the third or subsequent dose of the vaccine at the appropriate time for the natural course of the disease. Persistently elevated levels of specific IgG after vaccination, even at low levels, after COVID-19 infection suggests that SARS-CoV-2 IgG-specific antibodies help in monitoring humoral immunity for a long time. Crucially, however, they provide a new humoral response re-build up, observed twice following infection. Moreover, we have to add that in the five cases, we primarily saw good SARS-CoV-2 response seroconversion (IgM and IgG) after mild infection and some different responses after vaccination. A lack of a humoral-specific response for complete BNT162b2 vaccination and a booster (Case #2) does not mean seroconversion (S-RBD response). The mechanism of poor, late seroconversion is supposed to be involved in a substantial primary SARS-CoV-2 response (early responder). Moreover, a poor humoral response (seroconversion of IgG and IgM) was observed for the second and subsequent booster vaccinations in healthy SARS-CoV-2 asymptomatic cases (Case #1 and #3), not only for immunocompromised people.
P.K., S.S., and A.S.-B. analyzed the data and drafted the manuscript. M.J.B., D.E.K, R.M., A.H., and K.B. participated in data analysis and extensively reviewed the manuscript. D.E.K P.K., S.S., and A.S.-B. contributed to the clinical and laboratory data acquisition and reviewed the manuscript. All authors read and agreed to the published version of the manuscript.
The Bioethics Commission of the Medical University approved this research. Approval code: APK.002.267.2021; approval date: 29 April 2021.
Written informed consent was obtained from the patient(s) to publish this paper.
Data are available on request.
The authors thank the entire healthcare workers involved in the examinations at the Maria Sklodowska-Curie Bialystok Oncology Centre in Bialystok, Poland. All of the individuals included in this work consented to the acknowledgements.
The authors declare no conflicts of interest.
[Cross ref] [Google scholar] [Pub med]
[Cross ref] [Google scholar] [Pub med]
[Cross ref] [Google scholar] [Pub med]
[Cross ref] [Google scholar] [Pub med]
[Cross ref] [Google scholar] [Pub med].
[Cross ref] [Google scholar] [Pub med]
[Cross ref] [Google scholar] [Pub med]
[Cross ref] [Google scholar] [Pub med]
[Cross ref] [Google scholar] [Pub med]
[Cross ref] [Google scholar] [Pub med]
[Cross ref] [Google scholar] [Pub med]
[Cross ref] [Google scholar] [Pub med]
[Cross ref] [Google scholar] [Pub med]
[Cross ref] [Google scholar] [Pub med]
[Cross ref] [Google scholar] [Pub med]
[Cross ref] [Google scholar] [Pub med]
[Cross ref] [Google scholar] [Pub med]
[Cross ref] [Google scholar] [Pub med]
[Cross ref] [Google scholar] [Pub med]
[Cross ref] [Google scholar] [Pub med]
[Cross ref] [Google scholar] [Pub med]
[Cross ref] [Google scholar] [Pub med]
[Cross ref] [Google scholar] [Pub med]
[Cross ref] [Google scholar] [Pub med]
[Cross ref] [Google scholar] [Pub med]
[Cross ref] [Google scholar] [Pub med]
[Cross ref] [Google scholar] [Pub med]
Citation: Kosiorek P, Stróż S, Hryniewicz A, Kazberuk DE, Milewski R, Bartoszewicz K, Borkowska MJ, Stasiak-Barmuta A (2022) A New Set-Up of Vanishing Antibodies: A Biennial Follow-Up of Five Different Clients' Humoral Responses against SARS-CoV-2 after Systemic Vaccination in an Hospital In Poland. J Cancer Res Immunooncol. 08:156
Received: 30-Nov-2022, Manuscript No. JCRIO-22-20531; Editor assigned: 02-Dec-2022, Pre QC No. JCRIO-22-20531 (PQ); Reviewed: 16-Dec-2022, QC No. JCRIO-22-20531; Revised: 23-Dec-2022, Manuscript No. JCRIO-22-20531 (R); Published: 30-Dec-2022 , DOI: 10.35248/2684-1266.22.8.156
Copyright: © 2022 Kosiorek P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.