Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Research Article - (2020)Volume 11, Issue 5
Introduction: Collagen is a ubiquitous structural protein found in skin, bone, muscle and connective tissue in
humans and animals. Several studies have found significant positive impacts on skin health when collagen-containing
dietary supplements are routinely ingested. A newly developed collagen product (4Life Transfer Factor Collagen (TF
Collagen)) containing multiple types of collagen, transfer factors from colostrum, and other ingredients, was tested in vitro in a telomere model, and in vivo in a human skin health clinical study.
Objective: The purpose of these studies was to determine if TF Collagen supplementation could significantly
improve measures of aging and the overall skin health of the participants.
Methods: The in vitro telomere study measured telomerase activity of lung tissue cells over 15 passages by optical
density (OD). The telomerase activity was then compared between a control group and a TF Collagen-treated group
at corresponding passages. For the human study, 26 subjects (aged 35-55) consumed one packet (eight grams) of TF
Collagen daily for 84 days. Skin hydration, skin firmness, skin elasticity, and the appearance of fine lines and
wrinkles were measured at day 42 and day 84.
Results: The in vitro study found a significant increase in telomerase activity in cells treated with TF Collagen
compared to the control. In the human clinical study, TF Collagen significantly improved skin hydration, skin
firmness, and the appearance of fine lines and wrinkles, as well as subjective measures of skin health.
Conclusion: A multi-type collagen supplement with other ingredients activated telomerase in vitro and significantly
improved overall skin health in healthy men and women.
Collagen; Skin; Aging; Telomeres; Human
Collagen is a structural protein found in the skin, bones, muscles and other connective tissues of animals and humans. It is the most abundant protein in the animal kingdom and is fundamental to the strength and structure of the organism.
Collagen molecules pack together in helices, then fibrils, then fibers, and ultimately form connective tissue, whose purpose is to resist stretching. Collagen has greater tensile strength than steel on a weight basis.
There are 16 known types of collagen, though the majority are types I, II, and III [1]. Type I collagen forms a right-handed triple helix from two α-1 and one α-2 subunits. Each individual chain is 1050 amino acids long. Collagen has an unusual abundance of three amino acids: glycine, proline, and hydroxyproline, with hydroxylysine at the ends to connect strands together. Collagen is produced by connective tissue fibroblasts and some epithelial cells.
Collagen production decreases with aging, and some collagens become impregnated with calcium over time. This can result in lines and wrinkles in the skin, joint discomfort, more difficulty stretching, and other physical symptoms.
Telomeres are repeated DNA sequences (TTAGGG) located in the 5’ end of human chromosomes. These sequences lose up to 200 bases at the end of each DNA replication cycle [2]. The gradual loss of these repeats plays an important role in cellular death [3]. After a certain number of cell replications, the cell loses division capacity and undergoes apoptosis. Thus, telomere shortening controls the number of times one cell can divide, avoiding genetic aberrations (chromosome instability). Telomerase is the enzyme responsible for helping prevent the shortening of telomeres by continuously adding telomere repetitions to the end of telomeres [4].
Collagen may influence telomerase activity, so an in vitro study was included in the present study to test this hypothesis.
Schunck et al. [5] found that collagen supplementation may improve cellulite morphology in normal weight women. De Luca et al. [6] reported that a combination of marine collagen peptides plus several antioxidants improved skin elasticity, sebum production, and dermal ultrasonic markers in 37 to 72 years old healthy men and women (n=41). Bolke et al. [7] provided participants a product that contained 2.5 g of hydrolyzed collagen plus several other ingredients. They found the product improved skin hydration, elasticity, roughness, and density. A double-blind placebo-controlled clinical trial [8] of collagen ingestion during an eight-week period demonstrated that collagen hydrolysate consumption significantly improved facial skin moisture, elasticity, wrinkles and roughness. These results suggest that there is something unique about collagen consumption that contributes positively to skin structural maintenance over time.
The purpose of this study was to examine the effectiveness of 4Life Transfer Factor Collagen (TF Collagen). TF Collagen is a flavored drink mix dietary supplement containing collagen, transfer factors from colostrum, and other ingredients designed to be consumed by mixing with water. The goal was to study a complex matrix of ingredients for comparison to previous studies that have explored collagen alone, as well as with other ingredients, on measures of skin health [5-8]. An extensive literature review was completed and published in order to consider potential ingredients for this product [9]. To determine its effect on anti-aging parameters, TF Collagen was studied in vitro, to assess telomerase activity, and in a human clinical trial. It was hypothesized that TF Collagen supplementation would improve telomerase activity in vitro and result in positive measures of skin health for the human participants.
Product
The food supplement under investigation is 4Life Transfer Factor Collagen (TF Collagen), sold by 4Life Research USA, LLC. Product directions state to use one (1) packet per day by mixing it into eight (8) ounces of water. Each packet contains vitamin A (300 μg), vitamin C (270 mg), vitamin E (45 mg), biotin (500 μg), 4Life Tri-Factor Formula (100 mg), a collagen complex (3 g) containing hydrolyzed fish collagen (source of type I enzymatically hydrolyzed collagen with average molecular weight of ≤ 2000), chicken bone broth (source of type I, II, and III heat-hydrolyzed collagen), and egg shell membrane (source of type I, V, and X unhydrolyzed collagen), and a blend (136 mg) containing wheat seed extract and astaxanthin. Various collagens from different sources were included in the product because of an interest in seeing how involving many types of collagen together would affect different areas of health such as skin and hair. All ingredients in the product have been shown to have positive effects either on skin health, immune health or both. All improvements or results from the study would be based on the combination of ingredients, or rather the product as a whole, and not attributed to a single ingredient.
Telomere study design and partner
Telomerase activity testing was done through BioSRQ, LLC (Sarasota, Florida). In order to measure telomerase activity, TF Collagen was tested on a primary human cell line (IMR90) derived from lung tissue, an established cell model of telomere shortening, of a 16-week-old female Caucasian fetus. The lung tissue cells that were used had a finite lifespan. Cells were treated with each compound for 15 passages. Quantitative results obtained from lower-number passages were emphasized more than results from higher number passages. A study by Esquenet et al. [10] suggested that the higher the passage number, the more likely that the cell culture will not as accurately represent the original cell culture and how it reacts in the presence of certain compounds.
Toxicity test
Dose toxicity studies were initially carried out using a modified lactate dehydrogenase (LDH) release assay [11,12] to determine the appropriate test dose. For the TF Collagen compounds, the subsequent dose used for the study was the highest dose without any evidence of toxicity.
Cell treatment and sample collection
Cells were treated with TF Collagen for 15 passages. Telomerase activity was compared among different passages to see how activity changed over time in the presence of the TF Collagen compound.
The Telo TAGGG Telomerase PCR ELISA (Roche Applied Science, Indianapolis, IN, USA) was then performed following the steps outlined in the PCR ELISA kit’s manual. The assay was performed on the samples collected at passage 2 (P2), passage 4 (P4), passage 6 (P6), passage 8 (P8), passage 10 (P10), passage 12 (P12), and passage 15 (P15) to investigate the telomerase activity profile over time.
Data analysis
The absorbance values are reported as the A450 nm reading against the reference wavelength A690 nm. Additionally, the mean of the absorbance readings of the negative controls are subtracted from those of the samples. The positive control requires that the absorbance reading should be higher than 1.5 A450 nm-A690 nm units after the 20 min substrate reaction. Statistically significant differences between different passages are defined as a p-value ≤ 0.05, determined by a two-tailed twosample t-test.
Clinical study design
The clinical study was run at BioScreen Testing Services Division (Phoenix, AZ) and completed within a three-month period (April 25, 2019 to August 6, 2019; including a five-day washout period and 84 days of product usage). The study was conducted in accordance with the International Conference of Harmonization Tripartite Guideline on Good Clinical Practice, applicable FDA regulations/guidelines set forth in 21 CFR Parts 11 and 50, and standard practices of BioScreen Testing Services. The product tested was TF Collagen. If absolutely necessary, participants were able to use method-validated supplemental topical products Neutrogena SPF 30 Ultra Dry Sheer Touch Broad Spectrum Sunscreen and Neutrogena Facial Cleansing Bar Soap, Fragrance Free. All other skin products that were previously being used by participants, were not allowed to be used during the study period. Test products were also reviewed and approved for use by the Regulatory and Safety representatives of 4Life Research.
Subjects
Men and women aged 35-55 years (n=26) who met inclusion and exclusion criteria were recruited for the study (Figure 1). The target for the study was no more than ten percent male study participants, with a final percentage of fifteen percent male. There were no restrictions on race or skin type. Subject compliance was obtained by a consent form from each volunteer prior to initiating the study. Each subject was assigned a permanent identification number and also completed an extensive medical history form. Confidentiality was maintained according to the requirements of the review board.
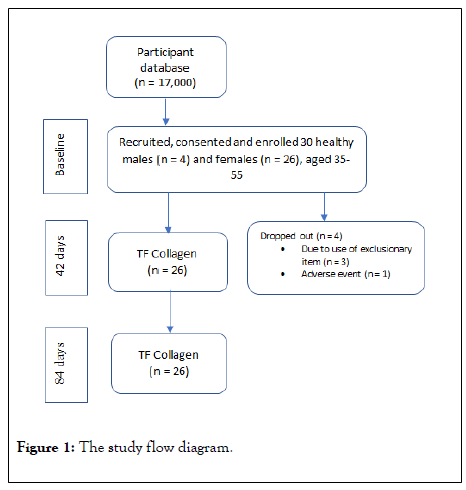
Figure 1: The study flow diagram.
Inclusion criteria
Healthy individuals who, at baseline, were free of any interfering dermatological or systemic disorder, and agreed to refrain from using personal care products except for those provided by BioScreen Clinical Services, Inc. (BCS). Individuals were also required to have visible global fine lines and wrinkles on the face.
Exclusion criteria
Individuals who had a history of any disease or were using immunosuppressive medications that could interfere with or increase the risk of study participation were excluded. Individuals with known allergies or sensitivities to product ingredients or with damaged skin at or in close proximity to test sites (e.g., sunburn, tattoos, scars, or other disfigurations) were also excluded.
Assessments
Evaluations of skin measurements, hair measurements, and self-reported improvements were taken at Day 0 (pre-treatment), Day 42, and Day 84 post-treatment intervals. Selection of assessments and instrumentation were determined and validated by Bioscreen Testing Services personnel. A survey of selfreported improvements was completed by each subject at baseline (pre-treatment), Day 42, and Day 84 post-treatment intervals.
Skin moisture
Skin moisture was measured by a Corneometer CM 825 (Courage and Khazaka, Germany) which determined the electrical capacitance of the skin in triplicate at each time interval. The measurement is made on the difference in the dielectric constant. When skin is hydrated, conductance and capacitance increase, and impedance decreases. Thus, an increase in electrical capacitance of the skin correlates with a higher level of skin hydration.
Skin firmness and elasticity
Skin elasticity and firmness were measured using a Cutometer MPA 580 (Courage and Khazaka, Germany). This device measures the viscoelastic properties of the skin through suction. A specified negative air pressure is applied on the skin drawing it into the aperture of a probe, and the depth of the skin penetration into the probe is measured. This measurement was taken at each time interval.
Fine lines and wrinkles
Evaluation of fine lines and wrinkles was accomplished through a validated clinical photography method. Photographs were taken in accordance with regulations of consumer protection agencies including the Federal Trade Commission, the Food and Drug Administration and other authorities. These guidelines were followed for all photos: 1) Test site position is the same, 2) Lighting conditions and distance from the camera is the same, 3) Same room and background is used, and 4) No jewelry is permitted. A blinded board-certified dermatologist for appearance of global fine lines and wrinkles evaluated the photos and ranked the test site of each subject using the following scale: 0 is none, 1-3 is mild, 4-6 is moderate, and 7-9 is severe. Half point increments were used in reporting results.
Hair diameter
Three hair samples were taken from each subject’s head at day 0, day 42, and day 84. Triplicate measurements of the hair diameter were recorded and change in average hair diameter over the testing period was calculated. On all strands, hair diameter was measured approximately 15 mm above the follicle using a Hitachi TM3030Plus Scanning Electron Microscope (Westford, MA, USA) with the assistance of the University of Utah ’ s Materials Characterization Lab (Salt Lake City, UT, USA).
Statistical analysis
Comparisons between pre-treatment values and post-treatment values of each tested parameter were analyzed statistically to determine differences, using a two-tailed p-value ≤ 0.05. A Student’s paired t-test was employed for normally distributed data, while the Wilcoxon Signed Rank Test was implemented for not normally distributed data.
For the questionnaires, the percentage of subjects responding in favor of the test product is reported. Statistical analysis was performed using a z-test, with statistical significance at a p-value ≤ 0.05.
In vitro telomerase assay
LDH assay: TF Collagen was tested at 200 μg/ml, due to toxicity at higher concentrations.
Telo TAGGG telomerase PCR ELISA assay: Passages 2, 8, 12, and 15 were selected to compare for telomerase activity because they represented telomerase activity from earlier passages to later passages with adequate spacing to allow for telomerase activity differentiation at different passages. The data showed that telomerase activity significantly diminished over time in the untreated control cells (See Control data in Figure 2). These results were expected and reflect telomere shortening in the primary IMR90 cells over their finite lifespan.
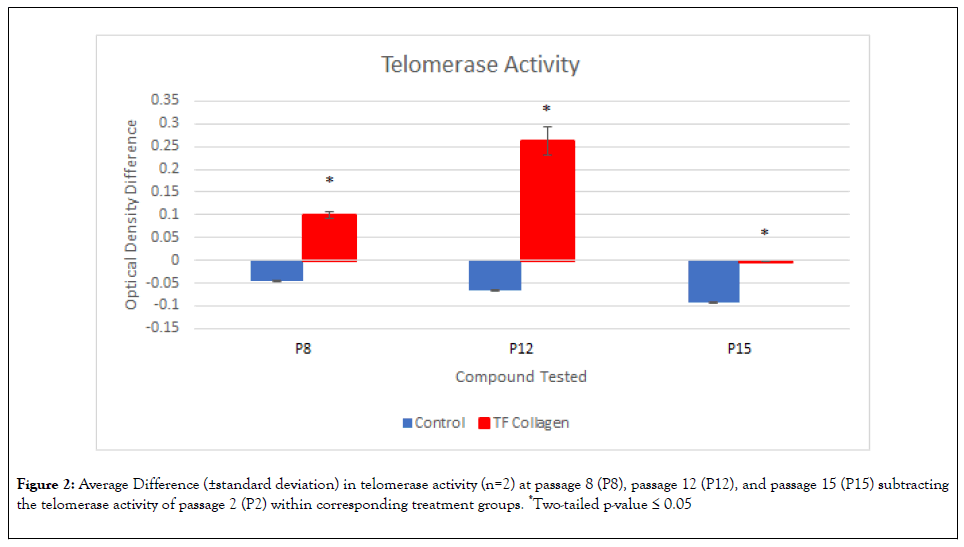
Figure 2: Average Difference (±standard deviation) in telomerase activity (n=2) at passage 8 (P8), passage 12 (P12), and passage 15 (P15) subtracting the telomerase activity of passage 2 (P2) within corresponding treatment groups. *Two-tailed p-value ≤ 0.05
When comparing the untreated control cells to the corresponding passages in the TF Collagen treated cells, P2 absorbance values were not significantly higher in the cells treated with TF Collagen. As the control cell telomerase activity decreased, the TF Collagen telomerase activity increased. Cell proliferation was much slower in the collagen group between P1 and P2 which could directly be seen in the obvious difference of P2 absorbance values of both treatments. Thus, P2 was used as the baseline value and subtracted from later passage values so the change in absorbance could be tracked during the subsequent passages. The change in TF Collagen telomerase activity was significantly higher than the telomerase activity change in the control group. Side by side comparisons of similar passage numbers in the collagen and control group resulted in a significantly higher change in telomerase activity for the TF Collagen group at P8 (p=0.002), P12 (p=0.004) and P15 (p=0.002) when compared to the control group (Figure 2). Interestingly, telomerase activity significantly decreased when comparing P12 to P15 within the TF collagen cells. The reason for the drop from P12 to P15 may be due to some toxicity observed over time that resulted in a slowing of cell growth or decreased cell responsiveness after a higher number of passages.
Human clinical study
Subject compliance and dropouts: Participants agreed to bring remaining product packets back at each measurement interval as evidence that they were consuming the product daily. Boxes containing packets had a checkbox for each day of the study, and participants would check the corresponding box after consuming the product. Boxes were brought back so that compliance could be measured based off the percentage of boxes that were checked. Percent compliance was 99.5%.
Only one participant dropped out during the study. The subject had been consuming the product for three days and claimed that it caused nausea. The subject voluntarily discontinued use of the product. The subject later indicated that after stopping consumption of the product, feelings of nausea subsided.
Results
Skin moisture: After 42 days of test product usage, 100% of subjects demonstrated a statistically significant improvement in skin hydration, with an average increase of 21% skin hydration. After 84 days of test product usage, 96% of subjects demonstrated a significant improvement with an average increase of 25% in skin hydration (Figure 3).
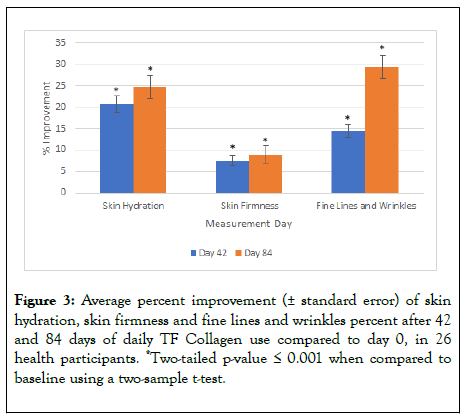
Figure 3: Average percent improvement (± standard error) of skin hydration, skin firmness and fine lines and wrinkles percent after 42 and 84 days of daily TF Collagen use compared to day 0, in 26 health participants. *Two-tailed p-value ≤ 0.001 when compared to baseline using a two-sample t-test.
Skin firmness and elasticity: After 42 days of test product usage, 96% of subjects demonstrated a statistically significant improvement in skin firmness, with an average increase of 7.5%. After 84 days, 88% of subjects demonstrated a significant improvement in skin firmness with an average increase of 8.9%.
Skin elasticity did not significantly change after 42 days or 84 days (data not shown) (Figure 3).
Fine lines and wrinkles: After 42 days of test product usage, 88% of subjects demonstrated a statistically significant improvement in appearance of global fine lines and wrinkles, with an average improvement of 15%. After 84 days of test product usage, 92% of subjects demonstrated a significant improvement in appearance of global fine lines and wrinkles, with an average improvement of 30% (Figure 3). An example set of images is shown in Figure 4 illustrating the effects seen in the study.
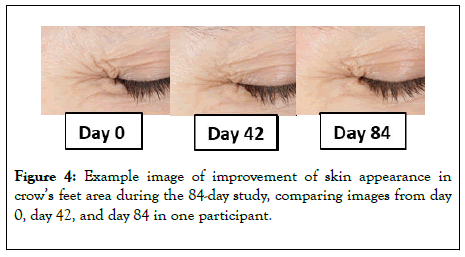
Figure 4: Example image of improvement of skin appearance in crow’s feet area during the 84-day study, comparing images from day 0, day 42, and day 84 in one participant.
Hair diameter: It was hypothesized that hair diameter might increase with collagen use. However, there was no significant change in average hair diameter at day 42 or 84 (data not shown). Figure 5 shows an SEM image of hair diameter that was taken during the analysis. Hair did not appear to be significantly impacted by TF Collagen.
Figure 5: SEM image of a strand of hair using a Hitachi TM3030Plus Scanning Electron Microscope.
Questionnaire: The pre-treatment questionnaire showed that 100% of participants viewed anti-aging as important, as well as the overall health of their skin, hair, nails, and joints. Subquestions were also asked in each category. Within the skin health sub-questions, 92% of the participants were specifically concerned with lines and wrinkles on their face, skin hydration, skin firmness, smoothness of their skin, and the brightness of their skin. Other sub-category question results are not included here. Day 42 and day 84 questionnaires (Figure 6) showed significantly favorable responses towards improvement in aging, skin health, hair health, and nail health. One of the primary functions of the product was not joint health, but participants perceived positive results in their joints, though that response was not statistically significant. Participants also reported positive views in sub-questions and towards using the product itself (data not shown).
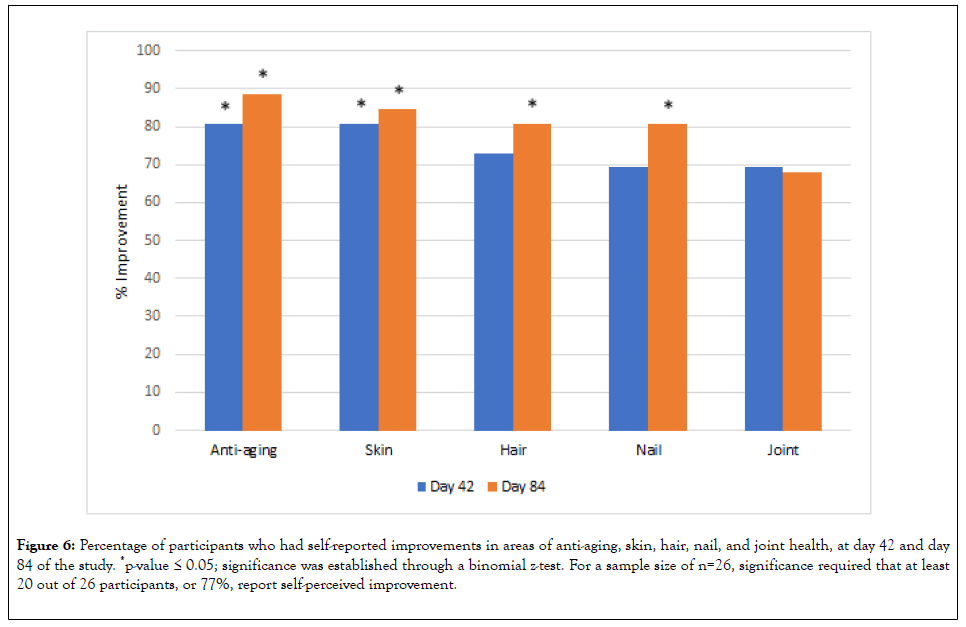
Figure 6: Percentage of participants who had self-reported improvements in areas of anti-aging, skin, hair, nail, and joint health, at day 42 and day 84 of the study. *p-value ≤ 0.05; significance was established through a binomial z-test. For a sample size of n=26, significance required that at least 20 out of 26 participants, or 77%, report self-perceived improvement.
Most survey responses (see Table 1) about use of the product mentioned that the product directions were easy to follow, and the smell and taste of the product was favorable, at both Day 42 and Day 84. Commentary about the effects of the product was generally very positive. Only day 84 responses are included in Table 1, as day 42 comments were similar.
| Participant ID | Day 84 | ||
|---|---|---|---|
| Please describe positive effect(s), if any, about this product | Please describe negative effect(s), if any, about this product. | Please provide additional comments, if any, about this product. | |
| 1440 | Easy to use. | None | N/A |
| 4576 | The collagen powder has a good taste. | N/A | N/A |
| 4607 | Healthier looking skin. Hydrated | taste too sweet gritty | |
| 5558 | |||
| 6158 | Easy to use. | Got tired of the same flavor every day | |
| 6576 | Easy to use and mix with water. Tasted good and liked how sweet it was. | NA | NA |
| 13704 | I had a little firmness and my dryness improve. | I didn’t see any improvement on my nails and hair. | |
| 15457 | Better overall appearance | ||
| 18647 | Thicker nail’s, Smoother skin. | ||
| 19270 | my nails grow a lot | None | my nails really never grow well they grew. |
| 20886 | Seems like my skin looks a little more hydrated. | N/A – none | |
| 21841 | I really like the product my skin looks really amazing my hair more healthier | N/A | I will really will like the know what this product is to recomend to every one and to buy. |
| 21988 | Improved my overall heath, my skin is soft all over. I look 5 years younger. Thank you please send life time supply. | No | Let me know when I can order! |
| 22844 | Softer skin | ||
| 23156 | Noticable improvements in skin health, hair is thicker | None | |
| 26000 | Noticeable difference in the stiffness of my hands in the morning | I didn’t notice anything negative besides the taste of the product. This might be better in capsule form | |
| 27107 | smooth skin fewer wrinkles smaller pores even skin tone nail growth | ||
| 27443 | Condition of skin is better, it is much smoother and softer | didn’t notice any | |
| 27537 | Easy to use. Quick and noticeable changes enjoyed this product. | N/A | |
| 28411 | Easy to take being all in me – once daily I feel like my face has more hydration. | only dissolves in warm liquid | I wish I could continue to take it. |
| 30159 | I’ve had much more glow to my skin. The tone has also improved. It has made my hair grow faster. I really enjoyed the benefits of this | None | |
| 30583 | it made me feel good about taking it and doing something good for my body. | None | N/A |
| 31519 | It was easy to remember to take in morning. Tasted good+I felt like I was healthier by drinking it | No negative effects | I’d definitely buy it if I had the opportunity. My face feels good + hydrated + I usually have pretty dry skin |
| 32008 | It was easy to use | tastes horrible | |
| 32895 | Noticed a difference in my skin while takin product firmness/elasticity – Healed a lot faster! | N/A | N/A |
| 32896 | It tastes good. Easy to dilute in hot water. | My lower back started to hurt and I’ve never had back problems. | I don’t know if I’d buy this. |
Table 1: Day 84 participant questionnaire comments. Subject comments are reported verbatim, including None, NA or N/A. Blank boxes means no response was provided. Only day 84 responses are included in Table 1, as day 42 comments were similar.
The telomerase activity study showed that a significant increase in telomerase activity occurred when the cell cultures were treated with the collagen supplement as compared to the untreated control group. When comparing the later passages P8, P12, and P15 of the collagen treatment group to corresponding passages in the control group, telomerase activity was significantly higher in the collagen treatment group at all three time points. An increase in telomerase activity is related to an increased lengthening of telomeres, whose shortening tends to occur during the aging process. A study by Tsoukalas et al. [13] saw similar results in the increase of telomerase activity when testing various natural compounds at various concentrations on human blood cells in vitro. Other studies [14,15] have shown increased telomerase activity in the presence of antioxidants, which are also present in TF Collagen in the form of multiple vitamins.
Results of the clinical human study statistically validated the claims that daily use of TF Collagen leads to improved skin hydration, improved skin firmness, and improved appearance of global fine lines and wrinkles in the participants. The results of our study were in line with other studies.
Inoue et al. [8] conducted a double-blind placebo-controlled study where 85 females were randomly assigned to ingest a five-gram sample containing a placebo (maltodextrin), or one of two collagen hydrolysates containing either a low ratio of dipeptide to product content (0.1 g per kg of product) or a high ratio of dipeptide to product content (2 g per kg of product) daily for eight weeks. Measurements of skin moisture, skin elasticity, and skin wrinkles and roughness were taken at baseline, after four weeks, and after 8 weeks. Results of this study showed a significant improvement in skin moisture and reducing wrinkles on facial skin, similar to our study. In a double-blind study performed by Kim et al. [16], 53 women with photoaged skin between the ages of 40 and 60 consumed 1000 mg of low molecular weight collagen protein (LMWCP) daily for 12 weeks. It was found that the oral intake of LMWCP improved the health of the tested subjects by significantly improving skin hydration, as well as skin wrinkling, after 12 weeks of intake. Skin elasticity, however, only improved in one measurement, but had no significant change in two other measured areas of skin elasticity. A 2019 study by Bolke et al. [7] focused on the significant improvements in 72 women aged older than 35 years who consumed a collagen product containing 2.5 g of collagen peptides, as well as other active vitamin ingredients, daily for 12 weeks in a randomized, placebo-controlled, blind study. Statistically significant improvements were found in skin hydration, elasticity, roughness, and density during the 12 weeks study. Genovese et al. [17] investigated the anti-aging properties of a liquid collagen supplement with 120 subjects over 90 days. Subject self-assessment questionnaires showed higher ratings of perceived skin health, yet results showed no significant difference in skin elasticity between treatment and placebo group.
One area we expected, but did not see a significant improvement, was in skin elasticity. Some studies did [7,16], while other did not or only showed improvements in certain aspects of skin elasticity [6,17]. There appears to be a unique relationship in skin between firmness and elasticity. In personal communication with Bioscreen testing services, this is a common pattern. Some products appear to push firmness higher, which was the case with our results. However, that can have a detrimental or nonsignificant effect on elasticity [6,17].
Hair follicle diameter was also not found to significantly increase during the 84 days study period. Biotin is included in the TF Collagen supplement, which has been connected to hair thickness, though is not yet proven to have efficacy for hair growth in healthy individuals [18]. One study [19] showed improvement in hair dullness and dryness with daily consumption of a collagen product that included hyaluronic acid, peptides, and lipids over the course of 60 weeks, with 54 women who had mild to moderate problems in skin, nail, or hair health. No specific studies on a healthy population measuring the direct relationship between hair health and collagen supplementation were found. Measurements of several markers for hair growth, in addition to hair follicle diameter, like the determination of hair density, dullness, dryness, or the anagen hair growth rate may be more appropriate for the evaluation of hair health in future studies.
Unlike most collagen products on the market, TF collagen contains five types of collagen, as well as supporting vitamins and botanical ingredients. The benefit of having five types of collagen is that different collagen types contain different amino acid structures and chain lengths and may play different roles in structural development and maintenance in the human body.
Significant improvements in general areas of skin health were seen in this study as well as the other mentioned studies. Other studies, however, focused on reporting results as average percent improvement only. The present study, in addition to this metric, also recorded percentage of subjects in the study that had significant results. For example, 100% of subjects showed significant improvement in skin hydration, approximately 90% of subjects showed significant improvement in skin firmness, and approximately 90% of subjects showed significant improvement in global fine lines and wrinkles.
In addition to objective skin measurements, post-treatment questionnaires that were filled out following day 42 and day 84 of supplement usage showed significant percentages of subjects with favorable responses in areas of improvement of anti-aging, skin health, hair health, nail health, and joint health. This gives insight and support into more practical perception aspects of research on this product that informs us about consumer acceptance.
Additional studies could be done to objectively study the effects of TF Collagen on hair health, fingernail health, and joint health. Results of this study could also be further validated by including a placebo group and making it a double-blind experiment.
Consistent and continuous intake of TF collagen over the course of 84 days has the potential to improve various aspects of skin health in healthy adults between the ages of 35-55. Testing after 42 days of daily intake of the TF collagen supplemental powder resulted in a significant improvement of skin firmness, skin hydration, and appearance of fine lines and wrinkles, and these significant improvements continued until the end of the study, at day 84. Participants reported observing these benefits through subjective surveys while taking the product. TF collagen could be of benefit to those desiring to improve their overall skin health through supplementation of a complex mixture of collagen types with other supporting ingredients.
We appreciate the services of BioSRQ and Bioscreen for their expertise in carrying out these studies.
All procedures carried out in this study were in accordance with applicable laws and IRB requirements, as stated in the Methods.
The authors are employees of 4Life Research USA, LLC.
This study was funded by 4Life Research.
VW and DV designed and managed the study. GL and TP analyzed the data, and drafted and edited the manuscript. All authors reviewed and approved the final manuscript.
Citation: Lee G, West V, Parker T, Vollmer D (2020) A Multi-Type Collagen-based Drink Supplement Significantly Improved Markers of Aging, both in vitro and in a Human Clinical Study. J Clin Exp Dermatol Res. 11:531. DOI: 10.35248/2155-9554.20.11.531
Received: 29-Jul-2020 Accepted: 12-Aug-2020 Published: 19-Aug-2020 , DOI: 10.35248/2155-9554.20.11.531
Copyright: © 2020 Lee G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.