Journal of Glycobiology
Open Access
ISSN: 2168-958X
ISSN: 2168-958X
Research Article - (2019)Volume 8, Issue 1
Diabetes is a widely existing disease in patients at risk of pancreatic cancer. It originates from pancreatic endocrine gland whereas pancreatic cancer develops from exocrine glands. Leukocyte cell surface glycans are involved in leukocyte-endothelial cell adherence and retinal endothelial cell death in diabetic retinopathy. A stimulation of hexosamine biosynthesis pathway occurs in diabetes and in pancreatic cancer controlled by oncogene KRAS variant. We examined 5 pancreatic non-tumor and 14 pancreatic tumor tissue specimens for quantitative changes in glycosyltransferse (GTs) activities in pancreatic tumorigenesis by following the incorporation of 14C or 3H monosaccharide (CPM) into specific acceptor catalyzed by 1 mg protein of Triton X-100 solubilized tissue extract. As compared to pancreatic non-tumor tissue specimens with a very low level of GTs activities, pancreatic tumor specimens on average contained 26.0, 42.9, 331.7, 121.0 and 62.8-fold of α1-2, α1-3, α1-4, α1-6 FTs and FTVI activities respectively. The major sialyltransferase α2-3 (O)ST and sialomucin glycoproteins increased 95.4 and 4.0-fold; N-glycan αMan: β1-2GlcNAc-T, chain elongating βGal: β1-3 GlcNAc-T and N-glycan GalNAc capping β1-3/1-4 GalNAc-T were respectively 95.0, 2.7 and 14.8-fold and the mRNAs of FUT-4, β1-3 and β1-4 GalNAc-Ts were 8.3, 12.0 and 2.4-fold respectively.
The increase in activity in neutrophils of retinopathy vs. normal was: β1-2-GlcNAc-T (9.0 fold), β1-3-GlcNAc-T (2.5), α1-3- FT (3.5), α1-6-FT (3.3), FTVII (1.9), αGalNAc: β1-3-GalT (1.4), βGlcNAc: β1-4-Gal-T (2.1), α2-3-(O)ST (2.1), α2-3-(N)ST (4.5), α2-6-(N)ST (8.1). GalNAc replacing Gal in LacNAc terminals results in changes of glycosyltransferase specificities and the modified GalNAc β1-4GlcNAc by FTs and STs bind to lectins such as WGA. In contrast to a low-level expression-difference of glycosyltransferases between tumor and non-tumor specimens from stomach, prostate and colon, a multifold increase in GTs in pancreatic tumor would indicate their significant role in invasion and intractability of pancreatic cancer.
Pancreatic cancer; Tumor specimens; Diabetic retinopathy neutrophils; N-and O- glycans, over-expression; Glycosyltransferase activities
Diabetes is a metabolic disease associated with the endocrine glands of pancreas and is a widely existing disease in patients at risk of pancreatic cancer. Diabetic retinopathy is a progressive vision threatening complication of diabetes [1]. Increased leukocyteendothelial cell adhesion appears to be a mechanism for capillary occlusion in diabetic retinopathy [2]. O-linked oligosaccharides on the surface of leukocytes play a crucial role in leukocyte-endothelial cell adherence through adhesion molecules such as selectins and integrins [3,4].
High glucose driven metabolic dysfunction such as increased involvement of the hexosamine pathway, accumulation of advanced glycation end-products and the induction of chronic low grade inflammatory signaling in the retina play an important pathological role [5]. Two types of glycosylation reaction namely O-GlcNAc [6] and β1-4GlcNAc branch from Man in complex N-glycans [7,8] were shown to be involved in type 2 diabetes.
Pancreas is sandwiched adjacent to stomach, gall bladder and small intestine and it lies in proximity to nearby blood vessels. Hence pancreatic tumor can spread quickly into other organs. Further many pancreatic cancers are encased in main blood vessels passing through pancreas and so surgical intervention is rather difficult. Pancreatic ductal adenocarcinoma (PDA) which develops from exocrine glands of pancreas is a very lethal cancer with a 5-year survival rate of ~5% [9].
Malignant progression proceeds with the early acquisition of activating mutations in the KRAS oncogene, which occurs in >90% of cases, and subsequent loss of tumor suppressors including INK4A/ARF, TP53, and SMAD4 [10]. KrasG12D serves a vital role in controlling tumor metabolism through stimulation of glucose uptake and channeling of glucose intermediates into the hexosamine biosynthesis (HBP) and pentose phosphate pathways [11]. A transgenic model of PDA showed that the inhibition of autophagy in presence of Kras and Trp53 mutations promotes cancer formation [12]. Several studies indicate that glycans and glycosylation of cellular proteins participate in the process of cancer cell adhesion, dissemination and metastasis [13-26].
As KrasG12D mediates pancreatic tumor maintenance by stimulating hexosamine biosynthesis pathway (HBP) and associated protein glycosylation [11], it is pertinent to ascertain whether the stimulation of HBP leads to overexpression of any specific or several glycosyltransferase activities and glycans in pancreatic tumor tissues. The present study examined the levels of several glycosyltransferase activities, their mRNA in some cases and sialylated O-glycans in tumor and non-tumor pancreatic tissue specimens and found their multifold overexpression in pancreatic tumor. Several glycosyltransferase activities were also found at higher level in neutrophils of diabetic retinopathy patients. The unprecedented exorbitant increase in the capacity of glycosylation could lead to detrimental biological consequences.
Tissue specimens
Human pancreatic and prostate tumor and non-tumor tissue specimens were obtained from pathology after surgical procedures at Roswell Park Cancer Institute and stored frozen within 1 h at 70°C. It is to be noted that in pancreatic cancer unlike many other cancers, there is a paucity of pancreatic tumor and non-tumor specimens from the same patients available for study. We studied the pancreatic tumor as well as non-tumor tissue specimens from the same patient in three pancreatic carcinoma cases (1T, 1N; 2T, 2N; 3T, 3N) and 11 pancreatic tumor specimens in which case normal pancreatic tissue specimens (6T-16T) were unavailable.
Two non-tumor pancreatic tissue specimens, for which the corresponding tumor specimens (4N, 5N) were unavailable, were also used. The prostate tumor and non-tumor tissue specimen from the same patient in nine prostate cancer cases (1T, 1N-9T, 9N) and three other available prostate tumor tissues (10T-12T) were used in this study for the purpose of comparison with pancreatic tumor data. When the tissue samples were collected from Pathology, a portion of the sample used for enzyme assay was fixed in formalin and embedded in paraffin. Slides were prepared from the paraffin block and stained with hematoxylin-eosin by standard procedures. A board-certified pathologist studied the slides to determine the distribution of cell types within the tumor and non-tumor tissues. The tumor tissue contained malignant epithelial cells while the non-tumor did not.
The pathology report containing pancreatic cancer diagnostic details and the amount of tissue specimens available for our studies is presented in Table 1. A comparison of each glycosyltransferase activity per mg protein of the Triton X-100 solubilized extract of tumor specimens with that of non-tumor specimens was made as it is relevant for understanding the quantitative change of each enzyme activity in tumorigenesis.
| Case | Site | Histology | Grade | Tissue (mg) |
|---|---|---|---|---|
| 14490 | Tail of Pancreas | Mucinous cystadenocarcinoma | Well differentiated | 1T: 960 |
| 1N: 560 | ||||
| 14596 | Tail of Pancreas | Infiltrating duct CA | Moderately differentiated | 2T: 715 |
| 2N: 2290 | ||||
| 15667 | Head of Pancreas | Infiltrating duct CA | Moderately differentiated | 3T: 740 3N: 860 |
| 15770 | Ampulla of Vater | Ampullary adenocarcinoma | Poorly differentiated | 4N: 215 |
| 15788 | Ampulla of Vater | Ampullary adenocarcinoma | Moderately differentiated | 5N: 715 |
| 15291 | Head of Pancreas | Infiltrating duct CA | Poorly differentiated | 6T: 150 |
| 15647 | Head of Pancreas | Infiltrating duct CA | Moderately differentiated | 7T: 410 |
| 15655 | Other specified parts of Pancreas | Infiltrating duct CA | Poorly differentiated | 8T: 350 |
| 11911 | Ampulla of Vater | Ampullary adenocarcinoma | Moderately differentiated | 9T: 220 |
| 12459 | Head of Pancreas | Infiltrating duct CA | Poorly differentiated | 10T: 160 |
| 12461 | Distal Pancreas | Mucinous adenocarcinoma | Moderately differentiated | 11T: 770 |
| 12876 | Head of Pancreas | Infiltrating duct CA | Moderately differentiated | 12T: 620 |
| 12956 | Ampulla of Vater | Ampullary adenocarcinoma | Moderately differentiated | 13T: 350 |
| 13767 | Junction of Ampulla and Duodenal mucosa | Intestinal type adenocarcinoma | Moderately differentiated | 14T: 290 |
| 12573 | Head of Pancreas | Adenocarcinoma | Poorly differentiated | 15T: 310 |
| 12584 | Head of Pancreas | adenocarcinoma | Poorly differentiated | 16T: 280 |
|
N: Non-tumor; T: Tumor. |
||||
Table 1: Pancreatic cancer diagnostic details of the patients and tumor and non-tumor tissue specimens.
Acceptor compounds
The synthetic compounds and modified glycopeptides used as acceptors in this study as shown in Table 1 have already been reported in our earlier studies [27-32] and, thus, are well-documented acceptors for measuring the reported enzyme activities.
Processing of tissue specimens
The tissues were homogenized at 4°C with four volumes (4 mL/g tissue) of 0.1 M Tris Maleate pH 7.2, 0.1% NaN3 using kinematica. After adjusting the concentration of Triton X-100 to 2% by adding 20% Triton X-100, these homogenates were mixed in the cold room for 1 h using Speci-Mix (Thermolyne) and then centrifuged at 20,000 g for 1 h at 4°C. Protein in the clear fat-free supernatant was measured by micro BCA method and then stored frozen at -20°C until use. Aliquots of 10 μL from this extract were used in most assays run in duplicates.
Glycosyltransferase activity in tumor lysate was determined by mixing the lysates with acceptor and radiolabeled monosaccharide donor under the reaction conditions detailed below, followed by separation of unreacted donor from the radioactive product using anionic or hydrophobic chromatography. In all cases, the radioactive content of isolated products was determined by using 3a70 scintillation cocktail (Research Products International, Mount Prospect, IL, USA) and a Beckman LS9000 scintillation counter. Controls for each assay contained the reaction mixture with everything except the acceptor. Radioactivity of product was subtracted from that of control to obtain the results presented in Figures and Tables. All assays were run in duplicate. We have taken care to ensure that the results from duplicate runs did not vary by more than 5%.
The following are the conditions for individual enzymatic assays. Reaction temperature in all cases was 37°C. α2-3- and α2-6 Sialyltransferase (ST) assay reactions proceeded for 2 h in a mixture containing 100 mM sodium cacodylate buffer (pH 6.0), 7.5 mM acceptor, CMP-[14C] NeuAc (0.05 μCi; 293 mCi/mmol Perkin- Elmer) and 10 μL cell extract in a total volume of 20 μL [30]. GlcNAc:1-4Gal-T and GalNAc:1-3Gal-T assay mixtures in duplicate contained 0.1 M Hepes–NaOH pH 7.0, 7 mM ATP, 20 mM Mn acetate, 1 mM UDP-Gal. UDP [14C] Gal (0.05 μCi; 327 mCi/ mmol; Amersham), 0.5 mM acceptor (unless otherwise stated) and 10 μL tissue extract in a total volume of 20μ L [27].
It was incubated for 4 h GlcNAc:1-3/4GalNAc-T assay mixtures in duplicate contained 0.1 M Hepes–NaOH pH7.0, 7 mM ATP, 20 mM Mn acetate. UDP [6-3H] GalNAc (0.20 μCi; 7.8 Ci/mmol: NEN-Dupont) 7.5 mM acceptor, 10μL tissue extract and incubated for 4 h [27]. α1-2-, α1-6-, α1-3- and α1-4-FTs assay reactions were carried out for 2 h in a reaction mixture containing 50 mM Hepes buffer (pH 7.5), 5 mM MnC12, 7 mM ATP, 3 mM NaN3, 3 mM synthetic acceptor or 40 μg of IgG glycopeptide acceptor, 0.05 μCi GDP-[U-14C] Fuc (290 mCi/ mmol; NEN-Dupont) and 10 μL tissue extract in a total volume of 20 μl [26,28,29]. GlcNAc-T assay mixture (20μL) in duplicate contained 70 mM Hepes-NaOH pH 7.0, 7mM GlcNAc 1,5 lactone, 14mM Mn acetate, 5mM ATP, 0.05μCi UDP [14C] GlcNAc (200 mCi/mmol; Amersham), 5 mM synthetic acceptor or 40 μg Fetuin glycopeptide acceptor and 10 μL tissue extract and incubated for 4 h at 37ºC [32].
Dowex-1-Cl or Sep-Pak C18 cartridges were used to isolate radiolabeled product from the reaction mixture. For α1-6 FT and α Man: β1-2 GlcNAc-T assays, the incubation mixture was diluted with 1 ml water and passed through a 1 ml bed volume of Dowex- 1-Cl column [26,27]. The column was washed twice with 1 ml water. The breakthrough and the water wash contained the [14C] fucosyl or [14C] GlcNAc-yl products formed from glycopeptide acceptors. About 3 ml of 0.1 M NaCl was used to obtain [14C]- fucosylated products from sialylated acceptors after water elution. For other glycosyl transferase assays, the radioactive products from benzylglycosides were separated by hydrophobic chromatography on Sep-Pak C18 cartridge (Water, Milford, MA, USA), and elution of the product was done with 3 ml methanol [30].
Reverse-exchange sialylation of sialomucin glycoproteins in pancreatic tissue specimens
We have well documented reverse sialylation activity of ST3 Gal II [α2-3(O)ST] which converts CMP to CMP-NeuAc using specific donor NeuAc α2-3 Gal β1-3 GalNAcα units [33, 34]. Radiolabeling of sialic acid in NeuAc α2-3 Gal β1-3 GalNAc units of several glycoconjugates were shown by using ST3 Gal II and CMP [9-3H or 14C] NeuAc utilizing the slow natural breakdown of CMP NeuAc into CMP and NeuAc at 37º C. The Triton X-100 solubilized tissue extracts (1N, 1T, 2N, 2T, 3N, 3T, 4N, 5N, 8T, 11T, 12T and 13T; 0.1mL each) were incubated separately at 37ºC for 20 h in0.1M Na cacodylate pH 6.0, 0.2 μCi CMP-[14C] NeuAc, and 25mU ST3Gal II (reaction volume 0.16ml). After incubation the reaction mixtures were diluted with 1.0ml water and dialyzed in the cold room against 2L of deionized distilled water with four changes for 72 h, lyophilized to dryness and then picked up in 1.0 ml water containing 0.2% Triton X-100. Incorporation of [14C] NeuAc per mg protein of these extracts was determined.
Isolation of neutrophils from control and diabetic retinopathy patients
Human polymorphonuclear leukocytes were isolated from freshly collected blood obtained by venipuncture in 10 U/ml heparin (Elkins- Sinn, Cherry Hill, NJ, USA). After gradient separation, erythrocytes were removed by hypotonic lysis. Polymorphonuclear leukocytes were then stored in Ca2+-free HEPES buffer at 4ºC. Cell viability was >99% and >90% of the isolated leukocytes were neutrophils. ~50 × 10</ neutrophils were further purified by sorting, using a FACS-Vantage instrument [35, 36]. Such samples were obtained from normal/control individual and diabetic retinopathy patients using protocols approved by the University at Buffalo Health Sciences IRB.
Quantitative reverse transcriptase-polymerase chain reaction (RTPCR) analysis
Total RNA isolation from tissue specimens, quantitative RT-PCR and quantification of mRNA levels of glycosyltransferase genes FUT 4, β1-3 GalNAc-T, and β1-4 GalNAc-T were performed as described before [37].
Evolution has selected the most diverse molecules such as glycoproteins containing complex glycan structures as the communication interface between cells and extracellular environment [38]. The early studies indicated the apparent role of glycans in cancer by showing that altered glycosylation on the surfaces or secreted proteins of tumor cells is common in pancreatic cancer and is thought to promote cancer progression [39,40]. Antibody-glycan microarray method found pro-inflammatory stimuli to alter the expression and glycosylation of mucins MUC 1, MUC 5AC and MUC 16 in multiple pancreatic cancer cell lines [41]. Since pancreatic tumor cells are usually part of an inflammatory environment, they are exposed to a variety of cytokines and growth factors [41]. It appears that the emergence of particular glycan structures on these cells may be functionally important in cancer progression [41]. Haptoglobin, leukemia-inhibitory factor receptor, centrosome-associated protein and vacuolar protein sortingassociated protein in pancreatic cancer sera were shown to express N-glycans containing core α1-6 Fuc, terminal GalNAc, α2-6 sialyl GalNAc and α1-2 Fuc as well as hybrid complex structures [42].
The present study used specific acceptors for assaying glycosyltransferase activities in tumor and non-tumor tissue specimens from pancreas and prostate cancer patients and also in normal and diabetic neutrophils as listed in Table 2.
| Glyosyltransferases | Acceptors |
|---|---|
| Fucosyltransferases | |
| α1-2- FTa | D-Fuc β1-3 GalNAcα-O-Benzyl (Bn) |
| α1-3- FT | 2-O-MeGalβ1-4GlcNAcβ-O-Bn |
| α1-4- FT | 2-O-MeGalβ1-3GlcNAcβ-O-Bn |
| FT VIb | GlcNAcβ1-4GlcNAcβ-O-Bn |
| α1-6- FT | Bovine IgG diantennary agalacto defucosyl glycopeptide |
| FT VII | NeuAc α2-3 Galβ1-4GlcNAcβ-O-Bn |
| Sialyltransferases | |
| α2-3 (O)ST | Galβ1-3 GalNAcα-O-Bn |
| α2-3 (N)ST | 4-O-MeGalβ1-4GlcNAcβ-O-Bn |
| α2-6 (N)ST | GalNAcβ1-4GlcNAcβ-O-Bn |
| α2-6 (O)ST | 3-O-MeGalβ1-3GalNAcα-O-Bn |
| βGal/GalNAc Transferases | |
| β1-4Gal-T | 3-O-MeGalβ1-3(GlcNAcβ1-6) GalNAcα-O-Bn |
| αGalNAc: β1-3Gal-T | 4-FluoroGlcNAcβ1-6 GalNAcα-O-Bn |
| β1-3/4GalNAc-T | GlcNAcβ-O-Bn |
| GlcNAc Transferases | |
| Man: β1-2GlcNAc-T | Fetuin triantennary asialo aglacto glycopeptide devoid of terminal GlcNAc residues |
| Gal: β1-3GlcNAc-T | Galβ1-4GlcNAcβ-O-Bn |
| aα1-2 fucosyltransferase acts on terminal βGal as well as on 6-deoxy βGal (D-fucose) as determined by using chemically synthesized compounds [28]; bOur earlier studies established that FT VI which is an α1-3-FT can also utilize GlcNAcβ1-4GlcNAc whereas α1-6- FT acts only on Asn-linked chitobiose attached to trimannosyl core [29,31]. |
|
Table 2: Acceptors used for assaying glycosyltransferases in tumor and non-tumor tissue specimens.
Fucosyltransferases in pancreatic cancer
The results presented in Figure 1 indicate that α1-2-, α1-3-, α1-4- FT and FT VI and α1-6-FT activities are either absent or very low in pancreatic non-tumor tissue specimens (Figure 1 A-E specimens 1-5). But all these FTs showed highly increased level of activities in all pancreatic tumor tissue specimens (Figure 1 A-E specimens 6-19) except for the tissue13T which expressed only α1-6FT at high level (Figure 1 E specimen 16) It is interesting to note that the increase in α1-3- and α1-4- FT activities are very high and multifold in all pancreatic tumor specimens except for 13T. FUT-4 mRNA (Table 3.) was very low in pancreatic non-tumor specimens in the range 0.04→0.15 whereas the pancreatic tumor specimens contained FUT-4 mRNA in the range 0.60→0.99.
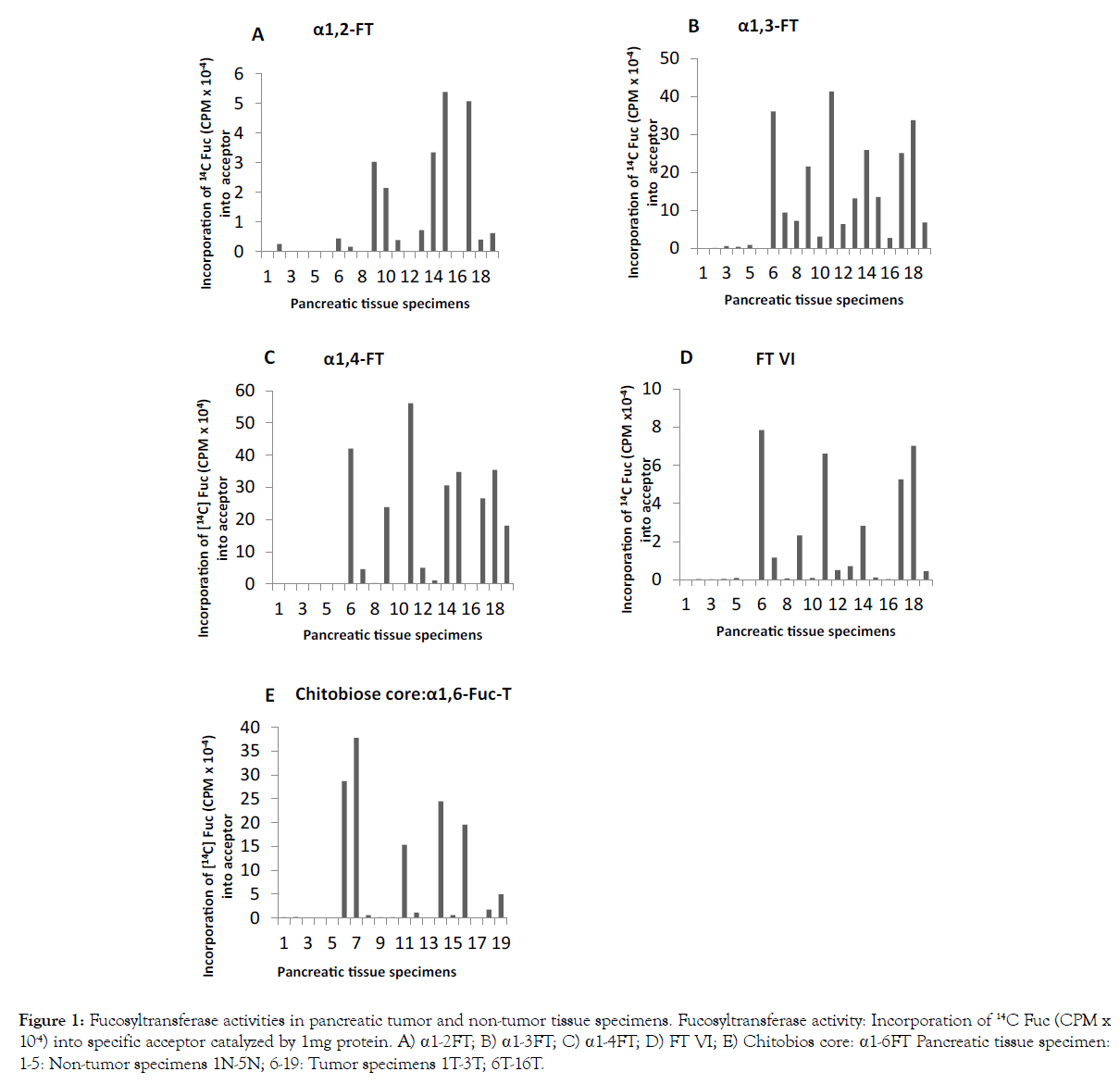
Figure 1: Fucosyltransferase activities in pancreatic tumor and non-tumor tissue specimens. Fucosyltransferase activity: Incorporation of 14C Fuc (CPM x 10-4) into specific acceptor catalyzed by 1mg protein. A) a1-2FT; B) a1-3FT; C) a1-4FT; D) FT VI; E) Chitobios core: a1-6FT Pancreatic tissue specimen: 1-5: Non-tumor specimens 1N-5N; 6-19: Tumor specimens 1T-3T; 6T-16T.
| Tissue Specimens | m-RNA FUT-4 | m-RNA β1-4 GalNAc-T | m-RNA β1-3GalNAc-T | |
|---|---|---|---|---|
| Pancreatic Normal | 1N | 0.04 | 1.95 | 0.20 |
| 2N | 0.15 | 2.91 | 0.49 | |
| 3N | 0.06 | 1.85 | 0.22 | |
| 5N | 0.04 | 0.80 | 0.05 | |
| Pancreatic Tumor | 1T | 0.60 | 5.34 | 0.75 |
| 9T | 0.99 | 10.10 | 11.03 | |
| 10T | 0.30 | 2.57 | 0.88 | |
| 11T | 0.40 | 5.31 | 2.05 | |
| 12T | 0.72 | 3.63 | 1.59 | |
| 13T | 0.47 | 2.02 | 0.96 | |
| 14T | 0.60 | 3.92 | 3.58 | |
| N: Non-tumor; T: Tumor | ||||
Table 3: m-RNA expression of FUT-4, β-1-3GalNAc-T and β-1-4GalNAcT in pancreatic tumor and non-tumor tissue specimens.
Sialyltranferases and sialomucin glycoproteins in pancreatic cancer
The data presented in Figure 2 indicate that all sialyltransferase activities are either absent or too low in pancreatic non-tumor specimens. The major sialyltransferase activity in pancreatic tumor is α2-3 (O)ST which forms NeuAc α2-3 Galβ1-3 GalNAc α-O-Ser/ Thr. Except for pancreatic tumor specimen 6T, 7T, 9T,10T and 14T (Figure 2 Specimens 9-11, 13 and 17) others exhibited multifold increase in α2-3 (O) ST activity. Pancreatic tumor α2-3 (N)ST activity was much lower and α2-6 (N) ST activity was minor as compared to that of α2-3 (O)ST.
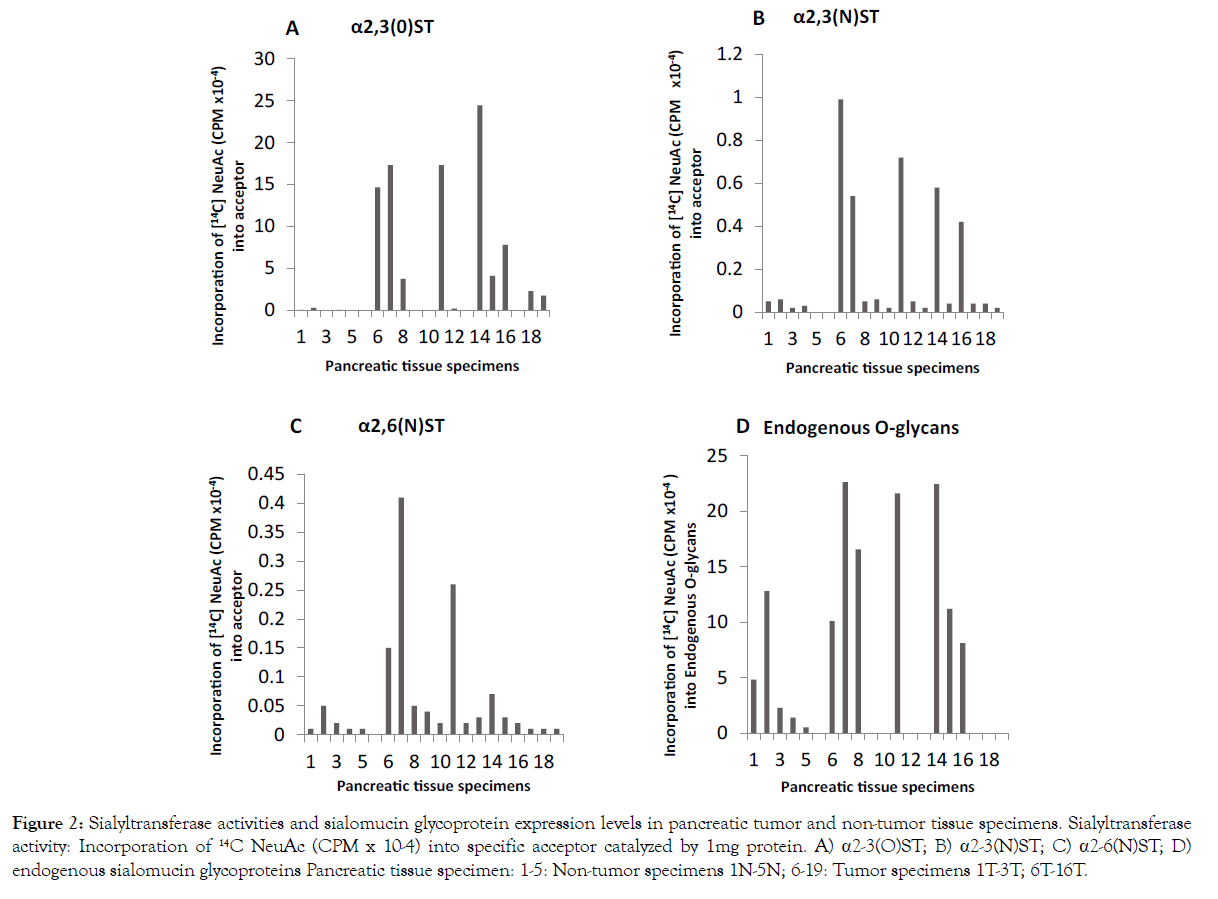
Figure 2:Sialyltransferase activities and sialomucin glycoprotein expression levels in pancreatic tumor and non-tumor tissue specimens. Sialyltransferase activity: Incorporation of 14C NeuAc (CPM x 10-4) into specific acceptor catalyzed by 1mg protein. A) α2-3(O)ST; B) α2-3(N)ST; C) α2-6(N)ST; D) endogenous sialomucin glycoproteins Pancreatic tissue specimen: 1-5: Non-tumor specimens 1N-5N; 6-19: Tumor specimens 1T-3T; 6T-16T.
An investigation on the level of sialomucin type glycoprotein in five non-tumor (1N-5N) (Figure 2 Specimens 1-5) and seven tumor (1T- 3T, 8T, 11T-13T) pancreatic tissue specimens (Figure 2 Specimens 6-8, 11, 14-16) using the technique of exchange sialylation showed a significant increase in sialomucin type glycoproteins in pancreatic tumor specimens as compared to non-tumor specimens examined. It is evident from the above results that pancreatic tumor contains an elevated level of sialomucin type glycoproteins and increased activities of α1-3- and α1-4-FTs and α2-3 (N) ST which favor the formation of Lewis blood group structures.
N-glycan associated glycosyltransferases in pancreatic cancer
As shown in Figure 3, N-linked complex-type chain initiating enzyme αMan: β1-2 GlcNAc-T, N-glycan chitobiose inner core α1- 6-FT (Figure 1 E), N-glycan chain elongating βGal: β1-3GlcNAc-T (Figure 3 D) and N-glycan GalNAc capping β GlcNAc: β1-3/β1-4 GalNAc-T (Figure 3 C) were found either absent or very low in non-tumor pancreatic tissue specimens (Figure 3 specimens 1-5). αMan: β1-2 GlcNAc-T in pancreatic tumor specimens except for 6T, 7T, 9T, 10T and 14T (Figure 3 E specimens 9, 10, 12, 13 and 17) showed increased activity, the increase being several fold in 1T, 2T, 8T, 11T and 13T (Figure 3 E specimens 6,7, 11, 14 and 16). Several fold α1-6-FT activity was found in tumor specimens 1T, 2T, 3T, 8T, 9T, 11T, 13T and 16T (Figure 1E specimens 6-8, 11, 12, 14, 16 and 19). It is noteworthy there is an inverse relationship between α1-2-FT and α1-6-FT; most of the tumors showing overexpression of α1-6-FT had low levels α1-2-FT (Figure 1A and 1E).
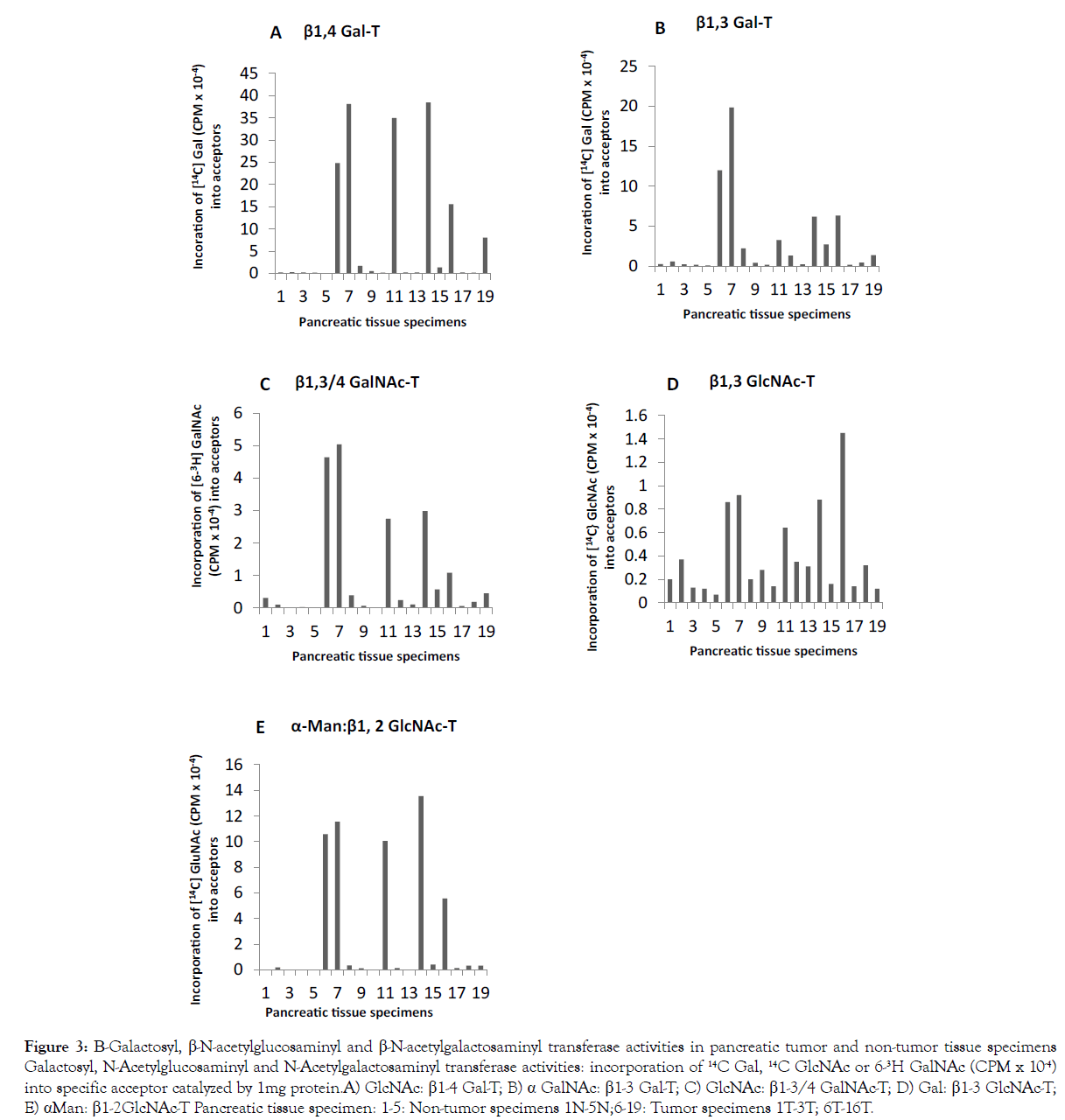
Figure 3:Sialyltransferase activities and sialomucin glycoprotein expression levels in pancreatic tumor and non-tumor tissue specimens. Sialyltransferase activity: Incorporation of 14C NeuAc (CPM x 10-4) into specific acceptor catalyzed by 1mg protein. A) α2-3(O)ST; B) α2-3(N)ST; C) α2-6(N)ST; D) endogenous sialomucin glycoproteins Pancreatic tissue specimen: 1-5: Non-tumor specimens 1N-5N; 6-19: Tumor specimens 1T-3T; 6T-16T.
Significant increase in β1-3 GlcNAc-T was seen with 1T, 2T, 3T, 8T, 11T and 13T (Figure 3 D specimens 6-8, 11, 14 and 16). β1-3/β1-4 GalNAc-T activity exhibited an increase of severalfold in pancreatic tumor specimens 1T, 2T, 3T, 8T, 11T, 12T, 13T and 16T (Figure 3 C specimens 6-8, 11, 14-16 and 19). These findings indicate an overexpression of N-glycan associated glycotransferases in pancreatic tumors. The levels of β1-4 GalNAc-T and β1-3 GalNAc-T mRNAs (Table 3) in pancreatic tumor specimens were much higher than that in non-tumor specimens. Range: β1-4 GalNAc-T N: 0.80→2.91 and T: 2.02/10.10; β1-3 GalNAc-T N: 0.05→0.49 and T: 0.75→11.03.
| Pancreas | Stomacha | Prostate | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tissue specimens | β1-3 Gal-T | β1-4 Gal-T | Tissue specimens | β1-3 Gal-T | β1-4 Gal-T | Tissue specimens | β1-3 Gal-T | β1-4 Gal-T | ||
| Incorporation of [14C] Gal (CPMx10-4) into the acceptor catalyzed by 1mg protein | ||||||||||
| 1N | 0.3 | 0.2 | 1N | 4.0 | 2.7 | 1N | 0.8 | 32.1 | ||
| 1T | 12.0 | 24.8 | 1T | 24.5 | 16.2 | 1T | 1.2 | 24.8 | ||
| 2N | 0.6 | 0.3 | 2N | 3.7 | 17.2 | 2N | 0.7 | 6.3 | ||
| 2T | 19.8 | 38.1 | 2T | 4.3 | 11.2 | 2T | 0.7 | 6.4 | ||
| 3N | 0.2 | 0.2 | 3N | 3.5 | 16.8 | 3N | 1.2 | 23.8 | ||
| 3T | 2.2 | 1.7 | 3T | 4.4 | 15.2 | 3T | 1.1 | 36.2 | ||
| 4N | 0.2 | 0.2 | 4N | 7.0 | 23.2 | 4N | 0.5 | 26.3 | ||
| 4T | 1.9 | 19.9 | 4T | 0.6 | 26.6 | |||||
| 5N | 0.1 | 0.1 | 5N | 10.2 | 27.5 | 5N | 1.6 | 40.2 | ||
| 5T | 3.6 | 25.8 | 5T | 1.3 | 37.9 | |||||
| 6T | 0.4 | 0.5 | 6N | 9.9 | 23.0 | 6N | 0.7 | 12.2 | ||
| 6T | 6.3 | 20.6 | 6T | 1.1 | 11.7 | |||||
| 7T | 0.2 | 0.2 | 7N | 6.7 | 25.5 | 7N | 1.6 | 16.9 | ||
| 7T | 6.6 | 40.4 | 7T | 1.4 | 73.5 | |||||
| 8T | 3.2 | 35.0 | 8N | 2.6 | 11.3 | 8N | 0.9 | 36.3 | ||
| 8T | 3.2 | 12.4 | 8T | 0.9 | 34.0 | |||||
| 9T | 1.3 | 0.2 | 9N | 8.8 | 36.3 | 9N | 0.8 | 34.4 | ||
| 9T | 13.8 | 21.1 | 9T | 0.7 | 13.7 | |||||
| 10T | 0.2 | 0.2 | 10N | 11.1 | 26.5 | 10T | 0.8 | 23.8 | ||
| 10T | 16.6 | 20.7 | ||||||||
| 11T | 6.2 | 38.5 | 11T | 10.3 | 9.3 | 11T | 1.0 | 42.2 | ||
| 12T | 2.7 | 1.4 | 12T | 2.4 | 6.2 | 12T | 0.7 | 30.7 | ||
| 13T | 6.3 | 15.6 | ||||||||
| 14T | 0.2 | 0.2 | ||||||||
| 15T | 0.4 | 0.2 | ||||||||
| 16T | 1.4 | 8.0 | ||||||||
| Mean Value | ||||||||||
| N | 0.3 | 0.2 | N | 6.8 | 21.0 | N | 1.0 | 25.4 | ||
| T | 4.0 | 11.7 | T | 8.2 | 18.3 | T | 1.0 | 26.7 | ||
| Fold | 13.3 | 58.5 | 1.2 | 0.9 | 1.0 | 1.1 | ||||
| N: Non-tumor; T: Tumor a In order to compare the values for gastric tissue specimens were used from an earlier report [43]. |
||||||||||
Table 4: A Comparison of the levels of αGalNAc: β1-3Gal-T and GlcNAc: β1-4 Gal-T activities in stomach, prostate and pancreatic normal and tumor specimens.
A comparison of pancreatic, gastric, and prostate tumors for mucin core 1 initiating enzyme αGalNAc: β1-3Gal-T and mucin core 2 elongating enzyme GlcNAc: β1-4Gal-T.
pancreatic non-tumor specimens as compared to the level of these enzymes in the non-tumor specimens from stomach [43] and prostate. Activity range: pancreas 0.1→0.3; stomach 2.7→36.3 and prostate 6.3→40.2. The β1-4 Gal-T activities range in tumor specimens were pancreas 0.2→38.5; stomach 6.2→40.4 [43] and prostate 6.4→40.2 (except 7T 73.5).
Hence the increase in β1-4 Gal-T activity level with respect to non-tumor tissue is multifold in pancreatic tumor as compared to that of gastric and prostate tumors. Core1 β1-3 Gal-T activity in both pancreas and prostate non-tumor specimens is much lower than stomach non-tumor specimens [43]. The β1-3 Gal-T activities range in non-tumor and tumor specimens are as follows: Pancreas N 0.1→0.6; T 0.2→19.8; stomach N 2.6→11.1; T 1.9→24.5 [43] and prostate N 0.8→1.6; T 0.6/1.4. It is clear from this data, the increase in β1-3 Gal-T activity is multifold in pancreatic tumor whereas there was no increase in prostate tumor specimens and less significant increase in gastric tumor specimens [43]. Thus, it becomes evident that both mucin β1-3 and β1-4 Gal-T activities which were quite low in normal pancreas were highly elevated in pancreatic tumorigenesis and this happening of a stimulation in the synthesis of mucin-type structures appears to be unique to pancreatic cancers due to over-expression of αGalNAc: β1-3Gal-T and core2 GlcNAc: β1-4 Gal-T.
Altering terminals in carbohydrate chains and its effects on gycosyltransferases activities and the binding of plant lectins
| Glycosyltransferases | Fucosyl and sialyl transferase activities: Incorporation of [14C] Fuc or [9-3H] NeuAc (CPMx10-4) into acceptor compounds | |||
|---|---|---|---|---|
| Fucosyltransferases | Galβ1-4GlcNAc β-O-Bn |
GalNAcβ1-4 GlcNAc β-O-Bn | Galβ1-3GlcNAc β-O-Bn |
GalNAcβ1-3GlcNAc β-O-Bn |
| FT IV (HL60 cell lysate) |
100.0% (4.69)a | 101.9% | 1.7% | 25.1% |
| α-1,4FT(FTIII) (purified from human lung tumor) | 12.0% | 51.2% | 100.0% (6.59) | 96.9% |
| Cloned FT V | 100.0% (2.53) | 109.4% | 90.1% | 88.0% |
| Cloned FTVI | 100.0% (5.27) | 97.4% | 5.0% | 93.8% |
| Sialyltransferases | ||||
| Cloned ST3Gal III | 100.0% (2.42) | 0.7% | 123.1% | 3.0% |
| Cloned ST6Gal I | 100% (4.02) | 65.2% | 3.0% | 8.1% |
| Plant Lectins | Binding characteristics of synthetic compounds | |||
| NeuAc α2-6 Gal β1-4GlcNAc | NeuAc α2-6 Gal NAc β1-4GlcNAc | NeuAc α2-6 Gal β1-4(6-O-sulfo) GlcNAc | ||
| SNA-1 | Regular binding | Regular binding | Tight binding | |
| WGA | Non-binding | Tight binding | ||
| Galβ1-4 (Fucα1-3) GlcNAc | GalNAcβ1-4 (Fucα1-3) GlcNAc | Gal/GalNAc β1-3 (Fucα1-4) GlcNAc | ||
| WGA | Non-binding | Tight binding | Non-binding | |
| aThe CPM for 100% activity is given in parenthesis for each enzyme; Note: α1-2 Fucosyltransferase, α1-3 Galactosyltransferase and 3-O-sulfotransferases act on Gal terminal but cannot act on GalNAc terminal. | ||||
Table 5: Biological consequence of sugar-alteration by showing the effect of GalNAc replacing Gal in LacNAc type 1 and type 2 carbohydrate Chain terminals on glycosylation and plant lectin-binding.
Table 5 presents the results from replacing Gal with GalNAc in LacNAc type I and type 2 acceptors. Remarkably, FT IV which is known to be strictly capable of carrying out α1-3 fucosylation only is shown here to be considerably active (25.1%) with GalNAc β1- 3GlcNAc β-O-Bn as compared to Gal β1-3 GlcNAc β-O-Bn (1.7%). The α1-4-FT from human lung [44] utilized both Galβ1-3 GlcNAc β-O-Bn (100.0%) and GalNAc β1-3GlcNAc β-O-Bn (96.9%) to the same extent whereas it acted more on GalNAc β1-4GlcNAc β-O-Bn (51.2%) as compared to Gal β1-4 GlcNAc β-O-Bn (12.0%).
Cloned FT V acted almost equally on type 1 and type 2 containing either Gal or GalNAc whereas FT VI utilized poorly Galβ1-3 GlcNAc β-O-Bn (5.0%) but acted on GalNAc β1-3GlcNAc equally well (93.8%) as on type 2 LacNAc containing either Gal or GalNAc (100.0% and 97.4%). Cloned ST3Gal III acted poorly on LacNAc type 1 and type 2 when Gal was replaced by GalNAc (3.0% and 0.7%). Further, it is noteworthy that Cloned ST6Gal I in contrast to ST3 Gal III acted fairly well on LacNAc type 2 containing GalNAc (LacdiNAc) (65.2%) whereas LacNAc type 1 containing Gal or GalNAc was a poor acceptor (3.0% and 8.1%).
The plant lectin SNA-1[45] bound comfortably well to α2-6 sialylated LacNAc type 2 containing either Gal or LacdiNAc GalNAc. In contrast, WGA did not bind to α2-6 sialylated LacNAc type 2 containing Gal but bound very tightly to α2-6 sialylated type 2 containing GalNAc β1-4GlcNAcβ sequence. It also exhibited non-binding to Lewis X structure but tight binding to GalNAc containing Lewis X structure. The above findings using some examples illustrate that GalNAC replacing Gal has distinct biological consequences and this kind of alteration may have an important role in pancreatic cancer.
Thus, the increased acceptor activity of FT III, FT IV and FT VI on GalNAc terminal LacNAc type 2 LacdiNAc (25.1%), GalNAc β1-3GlcNAcβ type 1 (51.2%) and GalNAcβ1-3GlcNAcβ type 1 (93.8%) respectively and almost equal activity of FT V on GalNAc terminal GalNAcβ1-3GlcNAcβ (88.0%), negligible activity of ST3 Gal III on GalNAc terminal Galβ1-3GlcNACβ and GalNAcβ1- 4GlcNAc (3.0% and 0.7% respectively) and moderate activity of ST6 Gal I on GalNAc terminal in GalNAcβ1-4GlcNAcβ (65.2%) would indicate that GalNAc replacing Gal in LacNAc terminals may play a significant role in pancreatic cancer invasion. Nonbinding of WGA to 6 sialyl Galβ1-4 GlcNAc and Gal β1-4 (Fuc α1-3) GlcNAc and tight-binding of WGA to 6 sialyl GalNAc β1-4 GlcNAc and GalNAc β1-4 (Fuc α1-3) GlcNAc [45] would suggest the utility of WGA as a tool for detecting pancreatic cancer.
A comparison of several glycosyltranferase activities in tumor and non-tumor tissue specimens from pancreas, gastric, prostate and colon cancers
The glycosyltransferase activities of tumor and non-tumor specimens from the same patient in 9 prostate cancer cases and 3 other prostate tumor specimens are presented in Table 6. The mean values of the folds of activities for tumor with respect to non-tumor specimens from pancreas, gastric, prostate and colon cancers are presented in Table 7.
| Tissue specimen | Incorporation of [14C] Fuc or [9-3H] NeuAc or [3H] GalNAc or [3H] GlcNAc (CPMx10-4) into the acceptor catalyzed by 1mg protein of TritonX-100 solubilized tissue extract | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| α1-2 FT | α1-3 FT | α1-6 FT | α2-3 (O)ST | α2-3 (N)ST | α2-6 (N)ST | βGlcNAc: β 1-3/4 GalNAc-T | αMan: β1-2 GlcNAc-T | ||
| 1N | 2.49 | 5.38 | 16.19 | 7.11 | 0.36 | 0.23 | 1.20 | 1.26 | |
| 1T | 1.58 | 7.17 | 13.96 | 10.31 | 0.98 | 0.60 | 1.84 | 0.94 | |
| 2N | ND | ND | 11.96 | 4.79 | 0.08 | 0.10 | 0.36 | 0.53 | |
| 2T | ND | ND | 12.92 | 6.87 | 0.11 | 0.10 | 0.51 | 0.63 | |
| 3N | 3.8 | 3.14 | 17.02 | 8.46 | 0.47 | 0.21 | 1.49 | 2.64 | |
| 3T | 4.65 | 3.82 | 9.50 | 5.90 | 0.71 | 0.41 | 2.25 | 1.54 | |
| 4N | ND | ND | 7.39 | 4.90 | 0.31 | 0.18 | 1.24 | 1.11 | |
| 4T | ND | ND | 1.46 | 9.30 | 0.48 | 0.35 | 1.13 | 2.33 | |
| 5N | 3.81 | 0.79 | 27.50 | 4.88 | 1.87 | 0.89 | 3.34 | 6.04 | |
| 5T | 4.46 | 1.94 | 22.02 | 3.11 | 0.72 | 0.22 | 3.60 | 1.00 | |
| 6N | 1.73 | 3.84 | 6.99 | 10.18 | 0.42 | 0.23 | 1.26 | 1.28 | |
| 6T | 3.94 | 2.73 | 7.20 | 6.74 | 0.36 | 0.30 | 1.48 | 0.48 | |
| 7N | 2.91 | 2.00 | 18.27 | 8.08 | 1.04 | 0.55 | 2.21 | 4.20 | |
| 7T | 6.87 | 5.73 | 9.79 | 13.41 | 4.54 | 3.50 | 5.74 | 2.37 | |
| 8N | 5.53 | 1.93 | 19.69 | 4.10 | 1.16 | 0.57 | 3.17 | 1.67 | |
| 8T | 4.83 | 1.69 | 6.04 | 2.48 | 0.70 | 0.37 | 2.54 | 2.00 | |
| 9N | 4.15 | 1.77 | 14.47 | 4.60 | 0.85 | 0.34 | 1.21 | 1.30 | |
| 9T | 1.48 | 1.72 | 8.36 | 3.61 | 0.65 | 0.24 | 3.05 | 2.10 | |
| 10T | ND | ND | 10.29 | 2.96 | 0.26 | 0.15 | 1.03 | 1.36 | |
| 11T | ND | ND | 21.26 | 20.27 | 1.18 | 0.71 | 1.68 | 3.17 | |
| 12T | ND | ND | 8.08 | 9.52 | 0.65 | 0.21 | 1.49 | 1.86 | |
| MV: | N | 3.49 | 2.69 | 15.50 | 6.34 | 0.73 | 0.37 | 1.72 | 2.23 |
| T | 3.97 | 3.54 | 10.91 | 7.87 | 0.95 | 0.60 | 2.20 | 1.65 | |
| Fold | 1.3 | 1.5 | 0.7 | 0.7 | 1.6 | 1.9 | 1.3 | 0.7 | |
| N: Non-tumor; T: Tumor; MV: Mean Value; Fold: Activity in tumor vs non-tumor | |||||||||
Table 6: Fucosyltransferases, sialyltransferases, GlcNAc: β1-3/4GalNAc transferases and αMan: β1-2 GlcNAc transferase activities in human prostate tumor and non-tumor tissue specimens.
| Mean value of the fold of activities in tumor vs non-tumor tissue specimens | ||||
|---|---|---|---|---|
| Glycosyltransferases | Pancreatic Cancera | Gastric Cancer b | Prostate Cancerc | Colon Cancerd |
| Fucosyltransferases | ||||
| α1-2FT | 26.0 | 1.2 | 1.3 | 9.0 |
| α1-3FT | 42.9 | 1.0 | 1.5 | 1.1 |
| α1-4FT | 331.7 | 4.7 | ND | 1.7 |
| FTVI | 62.8 | 27.4 | ND | 1.2 |
| α1-6FT | 121.0 | ND | 0.7 | ND |
| Sialyltransferases | ||||
| α2-3 (O)ST | 95.4 | 19.1 | 0.7 | 20.7 |
| α2-3 (N)ST | 9.3 | 2.3 | 1.6 | 8.7 |
| α2-6(N)ST | 4.0 | 9.9 | 1.9 | 19.5 |
| Gal/GalNAc-transferases | ||||
| GlcNAc: β1-4 Gal-T | 58.5 | 1.4 | 1.3 | 3.2 |
| αGalNAc: β1-3 Gal-T | 13.3 | 1.6 | 1.1 | 6.1 |
| GlcNAC: β1-3/4 GalNAc-T | 14.8 | 2.2 | 1.3 | |
| GlcNAc-transferases | ||||
| Gal: β1-3 GlcNAc-T | 2.7 | ND | ND | |
| αMan: β1-2 GlcNAc-T | 95.0 | ND | 0.9 | |
| ND: Not determined; a for the values of pancreas, refer Tables 2-5; bfor comparison purpose, the mean value of glycosyltransferase activity in gastric tumor and non-tumor specimens from the same patient (10 patients) were calculated from the data of an earlier report [43]; c for the values of prostate, refer Tables 5 and 7; d the fold of glycosyltransferase activity in colon tumor vs normal colon tissue was calculated from our previous data [31]. | ||||
Table 7: A comparison of the mean values of the fold of glycosyltransferase activities in tumor and non-tumor tissue specimens from pancreatic, gastric, prostate and colon cancers.
Mean values (fold of activity) for fucosyltransferases in the case of pancreatic tumor specimens are multifold higher when compared to gastric and prostate tumor specimens. In the case of FT VI only two gastric tumor specimens showed very high activities (51.4 and 199.5) that reflected in the high mean value (27.4). In the case of sialyltransferases, the mean values for pancreatic tumors are very much higher than that of prostate tumors. As only two gastric specimens contained high α2-3 (O)ST (7.9 and 172.0) and α2-6 (N) ST (7.3 and 82.2) activities, the mean values for α2-3 (O)ST (19.1) and α2-6 (N)ST (9.9) appear to be high reflecting these activities. Otherwise increase in fold of activity in pancreatic tumors is truly high as compared to gastric tumors. The activities of other enzymes Gal/GalNAc transferases and GlcNAc transferase have increased multifold in pancreatic tumor as evident from the mean values. All glycosyltransferase activities did not increase to a multiple level in gastric and prostate tumor specimens.
Neutrophil glycosyltransferase activities in diabetic retinopathy
The neutrophils were isolated separately from blood of five donors, one being a control individual and the other four diabetic retinopathy patients. The glycosyltransferase activities of these neutrophil samples were determined, and the results are presented in Table 8. Fucosyltranseferases α1-2 and α1-4 activities are absent in control neutrophils and either absent or in trace amount in diabetic neutrophils. α1-3-FT which forms Lewis X and FT VII which forms sialyl Lewis X from 3 sialyl Galβ1-4 GlcNAc are the major fucosyltransferase activities in neutrophils. α1-3-FT activity was almost 4-5-fold in diabetic neutrophils except for donor D (1.6 fold). FT VII activity was about 2-3-fold in diabetic neutrophil except for donor D (0.9 fold).
| Glycosyltransferase | Glycosyltransferase activities: Incorporation of [14C] - Fuc, Gal or GlcNAc or [9-3H] NeuAc [CPMx10-3] into the acceptor catalyzed by 1 mg protein of the neutrophil extract | |||||
|---|---|---|---|---|---|---|
| Neutrophil Donors | ||||||
| Control | Patients | |||||
| A | B | C | D | Mean Value | ||
| A. Fucosyltransferases | ||||||
| α1-2FT | 0 | 0.2 | 0.3 | 0 | 0 | |
| α1-3FT | 7.1 | 25.4 | 33.8 | 29.3 | 11.1 | |
| [3.6] | [1.6] | [4.1] | [1.6] | -3.5 | ||
| α1-4FT | 0 | 0.6 | 1.1 | 0.1 | 0 | |
| FTVII | 20.3 | 40.8 | 48.2 | 55.2 | 18.7 | |
| [2.0] | [0.9] | [2.7] | [0.9] | -1.9 | ||
| α1-6FT | 20.8 | 56.7 | 59 | 134.7 | 26.3 | |
| [2.7] | [1.3] | [6.5] | [1.3] | -3.3 | ||
| B. Sialyltransferases | ||||||
| α2-3 (O)ST | 13.8 | 28.4 | 23.8 | 38.9 | 26.3 | |
| [2.1] | [1.9] | [2.8] | [1.9] | -2.1 | ||
| α2-6 (O)ST | 0.8 | 1.9 | 1.5 | 2.1 | 1.6 | |
| [2.4] | [1.9] | [2.6] | [2.0] | -2.2 | ||
| α2-3 (N)ST | 1.7 | 5.8 | 5.5 | 13.1 | 5.9 | |
| [3.4] | [3.5] | [7.7] | [3.5] | -4.5 | ||
| α2-6 (N)ST | 0.7 | 4.6 | 4.1 | 9.1 | 4.8 | |
| [6.6] | [6.9] | [13.0] | [6.9] | -8.1 | ||
| C. Galactosyltransferases | ||||||
| β1-4 Gal-T | 173.9 | 328.8 | 335.1 | 506.4 | 288.8 | |
| [1.9] | [1.7] | [2.9] | [1.7] | -2.1 | ||
| αGalNAc: β1-3 Gal-T | 23.8 | 24.9 | 40 | 39.6 | 29.3 | |
| [1.1] | [1.2] | [1.7] | [1.2] | -1.4 | ||
| D. N-Acetylglucosaminyl-transferases | ||||||
| β1-3 GlcNAc-T | 83.6 | 156.8 | 197 | 261.7 | 197.7 | |
| [1.9] | [2.4] | [3.1] | [2.4] | -2.5 | ||
| αMan: β1-2 GlcNAc-T | 15.1 | 138.9 | 147.05 | 183.9 | 69.7 | |
| [9.2] | [4.6] | [12.2] | [4.6] | -9 | ||
| The fold of enzyme activity in patients with respect to that in control is shown in brackets. The mean value of enzyme activities in fold is shown in parenthesis | ||||||
Table 8: Glycosyltransferase activities of neutrophils isolated from the blood of control and diabetic retinopathy patients.
The major sialyltransferase activity is α2-3 (O)ST which is about 2-3-fold in diabetic neutrophils. The control neutrophils contained minor activities of other sialyltransferases α2-6 (O)ST, α2-3 (N)ST and α2-6 (N)ST but the diabetic neutrophils had about 2-3-fold, 3-8-fold and 7-13-fold activities of α2-6 (O)ST, α2-3 (N)ST and α2-6 (N)ST respectively. The core2 β1-4 Gal-T forming LacNAc type 2 which had high activity in neutrophils also exhibited an increase of activity (1.7/2.9-fold) in diabetic neutrophils. There was an appreciable increase in αGalNAc: β1-3 Gal-T activity in diabetic neutrophils (1.1→1.7-fold).
Among the glycosyltransferase activities associated with N-glycan biosynthesis, in the case of α1-6 FT which is also a major fucosyltransferase activity in neutrophils, an increase of 1.3→6.5- fold activity was found in diabetic neutrophils. The key enzyme in complex N-glycan biosynthesis namely αMan: β1-2 GlcNAc-T, there was multifold increase activity in diabetic neutrophils (4.6/12.2- fold). The complex N-glycan chain elongating enzyme Gal: β1-3 GlcNAc-T which is a major activity in neutrophils exhibited 1.9→3.1-fold increase in activity in diabetic neutrophils.
The findings of the present study validate the role of specific sets of enzymes that contribute towards the assembly of abnormally glycosylated molecules in pancreatic cancer progression. The overall profiling of glycosyltransferases (GTs) has been useful in identifying potential glycan structures altered in cancer [31]. A functional glycomics study pointed out that gene expression data would be more powerful when used in conjunction with biochemical data such as GTs activities [46]. A recent study found that changes in glycan structures, generally but not uniformly, correlate with alterations in transcript abundant for the corresponding biosynthetic enzymes [47].
Further, it is evident that a greater degree of regulation is necessary for the synthesis of distal branching and capping modifications of glycan chains as they are more accessible for mediating biological interactions in order to play regulation and functional roles in biological systems [47]. Cancer specific glycan expressions are better measured by following the GTs activities rather than the underlying transcription profile. Thus, it is apparently highly meaningful if cancer studies focus on GTs activities and related biochemistry rather than relying entirely on gene expression.
Fucosylation in pancreatic cancer
The present study found highly elevated level of fucosyltransferase activities in pancreatic tumor and this finding is quite consistent with the following earlier reports that fucosyltransferases regulate the synthesis of tumor associated carbohydrate determinants Lewis X, Lewis Y, sialyl Lewis X and sialyl Lewis a in pancreatic cancer [20]. A study on fucosyltransferase activities in human pancreatic tumor tissues and pancreatic tumor cell lines showed that FUT6 transcript was detected only in pancreatic cancer tissues but is not expressed in normal pancreas [21].
Increased branching of N-linked oligosaccharides and increased fucosylation and sialylation were observed in pancreatic cancer serum proteins [22]. Concentration of fucosylated haptoglobin increased in the sera of patients with pancreatic cancer compared to those of other types of cancer and normal controls [23]. Triantennary N-glycans containing a Lewis X –type fucose markedly increased at the Asn 211 site of haptoglobin N-glycan. Difucosylated tetra antennary N-glycans were observed only at this site in pancreatic cancer patients [23]. RNase 1 from healthy pancreatic cells contains neutral complex biantennary structures. In contrast, RNase1 glycans from tumor cells (Capan-1) were fucosylated hybrid and complex biantennary glycans with GalNAc- GlcNAc antennae [24].
Sialylation in pancreatic cancer
Cancer associated alteras largely occur from glycan branching of lactosamine chain in both N- and O- glycans [26]. One aspect of lactosamine extension is associated with formation of Lewis blood group structures such as CA 19-9 antigen which is a commonly used serum-based marker of pancreatic cancer [48]. A recently identified carbohydrate antigen sialyl-TRA in addition to CA 19-9 was shown to be an accurate serological biomarker of pancreatic cancer [49].
The attachment between PCI pancreatic carcinoma cells and activated endothelial cells is mediated by sialyl Lewis a in pancreatic carcinoma and E-selectin in endothelial cells [50,51]. The level of surface sialyl Lewis a expression of PCI cells correlates with a number of metastatic colonies in the liver [52]. Capan-1 and MDA Panc-3 cells contained RNase1 glycans with sialylated structures completely absent in the healthy pancreas [24].
SialylLea and integrin mediate the process from adhesion to implantation of human pancreatic cancer SW1990 cells to endothelial cells [13]. CD44 and integrin play important roles in the initial attachment of SW1990 cells to mesothelial cells [13]. SW1990 cell adhesion to E selectin is mediated by ligands on mucinous glycoproteins [14].
MUC4 mucin is aberrantly expressed in pancreatic tumors with no detectable expression in the normal pancreas [15]. A progressive increase of MUC4 expression in pancreatic intraepithelial neoplasia suggests its association with disease development [15]. Over- expression of MUC1 by tumor cells simultaneously mediates and blocks specific molecular interactions between exogenous cell ligands and cell surface receptors [16]. Muc1 is heavily glycosylated in normal epithelial but is overexpressed and differentially glycosylated in pancreatic cancer.
This altered glycosylation includes the shortened core-1 O-glycans for monosialyl and disialyl T-antigens [17]. In consistent with the findings of these earlier studies, the present study found that the major sialyltransferase activity in pancreatic tumor as α2-3 (O)ST implying predominant expression of sialyl T-antigen. Further a moderate level of α2-3 (N)ST activity and multifold overexpression α1-3 and α1-4 FT activities would favor the expression of sialyl Lewis X and Sialyl Lewis a in pancreatic cancer. In support of the high level α2-3(O)ST activity we have also shown in the present study a significant increase in the level of sialomucin glycoproteins containing sialyl T-hapten units in pancreatic tumor.
Asn-linked glycans in pancreatic cancer
Our present findings that the increase in the activities N-glycan associated glycosyltransferases are several folds in pancreatic tumor are supported by earlier studies showing the occurrence of Lewis X bearing triantennary and tetraantennary N-glycans in haptoglobin and triantennary glycans with GalNAc-GlcNAc antennae in RNase in pancreatic cancer [23,24].
There was a remarkable increase (40%) in core fucosylated biantennary glycans in the pancreatic cancer serum RNase 1, suggesting that there is a subset of tumor associated glycoforms of RNase1 [25]. Lectin antibody microarrays were utilized to detect unique glycosylation patterns of proteins in serum [53]. α1-β glycoprotein response to SNA resulted in specific detection of pancreatic cancer with high sensitivity and specificity [53]. The resulting scatterplots also showed the ability to clearly distinguish pancreatic cancer from chronic pancreatitis, diabetics or normal samples [53]. The response of protein amyloid to SNA also indicated its acceptable ability to detect pancreatic cancer [53].
Glycosyltransferases in neutrophils of diabetic retinopathy
The present study showed that the neutrophils from diabetic retinopathy patients contained a significant increase in glycosyltransferase activities of O-glycans biosynthesis and multifold increase in N-glycan biosynthesis associated glycosyltransferase activities. The glycosyltransferase activities of diabetic neutrophils indicate that α1-3-FT and FT VII forming respectively Lewis X and sialyl Lewis X, with the capability of producing N-glycans containing core α1-6 and elongated α1-3 fucosylated lactosamine chain are dominant in diabetic neutrophils whereas core1. GalNAc: β1-3 Gal-T and α2-3 (O)ST act together giving rise to sialyl T antigen.
Diabetic retinopathy is characterized by capillary occlusion, formation of microvascular lesions and retinal neovascularization adjacent to ischemic areas of retina [54, 55]. Studies using human tissue demonstrate a strong relationship between leucocyte endothelial cell adhesion and retinal capillary damage in diabetes [2]. The changes in the expression of O-linked oligosaccharides on the surface of leucocytes appear to be involved in the increased adhesion to endothelial cells [56]. Further carbohydrate composition changes of glycoconjugates constituting the glycocalyx of microvascular cells could be involved in the alterations of cell-cell interactions observed in diabetic retinopathy [57]. The metabolic dysfunction in diabetes includes increased involvement of the hexoamine pathway and accumulation advanced glycation end- products [5]. An increase in the level of O-GlcNAc in diabetes was found to lead to insulin resistance [6]. The MGAT4A gene that encodes the major Gn T-IV enzyme Gn T-IVa in pancreas is downregulated in diabetic patients [7,8]. Cell surface residency of the glucose transporter GLU T2 was impaired in Gn T-IVa deficient βcells [7,8].
P-selectin binds its main ligand PSGL-1 with high affinity and mechanical stability. E-selectin and L-selectin recognize many ligands with lower intrinsic affinity [58,59]. To compensate for their intrinsically low association rates, the E- and L- selectin determinants are thought to be presented in closely spaced clusters. N-glycan linked 6-Sulfo sialyl Lewis X has the critical function of L-selectin-dependent lymphocyte homing and recruitment [60]. A synthetic mucin core 2 compound GalNAcβ1-4 (Fucα1-3) GlcNAcβ1-6 (NeuAc α2-3 Galβ1-3) GalNAc α-OMe was ~ 6-fold better than sialyl Lewis X as inhibitor of L-and P- selectin binding [61].
PSGL-1 expressed on leucocytes is a high affinity ligand for E-, P-, and L- selectins. This glycoprotein has 71 Ser/Thr sites for O-glycans and 3 Asn sites for N-glycans. Sialyl Lewis X expressed on PSGL-1 represents the prototype oligosaccharides that bind selectins [36]. Using a CRISPR-Cas 9 tool kit to selectively truncate O-glycans, N-glycans and glycosphingolipids, it was shown that leucocytes rolling on P- and L- selectins is ablated in cells lacking O-glycans and an increased cell rolling velocity was found in cells with N-glycan truncation [62-71]. The present study showed a multifold increase in N-glycan associated glycosyltransferase activities and a significant increase in O-glycan associated glycosyltransferase activities in diabetic retinopathy neutrophils. Thus, it is apparent that diabetic neutrophils can bring adverse changes to leucocyteendothelial cell adhesion process due to their increased capacity of N- and O-glycans associated glycosylations.
It is evident from the present study that an exorbitant increase in the activities of the entire spectrum of glycosyltransferases we have examined is happening in pancreatic tumorigenesis. It is apparent that altered glycosylations on the surfaces and secreted proteins of pancreatic tumor cells may be the outcome of multifold increase in the level of N- and O-glycans associated glycosyltransferase activities in pancreatic tumor. The use of chemically synthesized well defined acceptors of enzymes leads to the discovery of new activities. α1-4 GlcNAc–capped O-glycans frequently expressed in pancreatic cancer cells indicated the use of α1-4 GlcNAc-T mRNA expressed in the mononuclear cell fraction of peripheral blood for the detection of pancreatic cancer.
We identified subsequently in gastric tumor αGlcNAc-T acting on terminal βGlcNAc in mucin core 2 trisaccharide GlcNAc β1-6 (Galβ1-3) GalNAcα- by showing the product to bind completely to PNA-agarose, its complete resistance to Jack bean β-N-acetylhexosaminidase and non-binding to PNA-agarose after recombinant β1-3-galactosidase treatment . If such α GlcNAc-T occurs in pancreatic tumor, it will be additionally useful for early detection and tracking of pancreatic cancer. Further, as shown in Table 8, pancreatic tumors express high levels of GlcNAc: β1- 3/4GalNAc-T. The appearance of β1-4GalNAc-T combined with high expression of α1-3-FT can generate GalNAcβ1-4 (Fucα1- 3) GlcNAcβ located on N-glycans as well as O-glycans. These glycans can bind to E-selctins which can lead to pancreatic cancer progression.
In contrast to normal colon, gastric and prostate tissues, normal pancreatic tissue is unique due to the fact that both N-glycan and O- glycan glycosyltransferase activities are very low, and this situation is akin to normal breast tissue . Pancreatic tumor cells are part of inflammatory microenvironment being exposed to a variety of cytokines and growth factors. Multiple mucin domains interact differently and regulate different components of the tumor micro environment . Several over-expressed mucins impede drug delivery to pancreatic tumors, suggesting that targeting mucin biosynthesis through mucin core 2 β1-6 GlcNAc-T (GCNT3) may improve drug responsiveness. Talniflumate alone and in combination with low dose gefitinib reduced GCNT3 expression leading to the disrupted production of mucins in vivo and invitro. Apparently certain glycan structures play a role in cancer progression by affecting tumor cell invasiveness, ability to disseminate through the blood circulation and to metastasize in distant organs.
During metastasis tumor cell-derived glycans enable binding to cells in their microenvironment including endothelium and blood constituents through glycan-binding receptors-lectins. Our earlier studies found a multifold elevation of Gal:3-Osulfotransferases (Gal3-sulfo-T2 and Gal3-sulfo-T4) in contrast to glycan:glycosyltransferases in breast, colon and gastric tumors indicating an apparent role of sulfated glycans and sufation process in the progression of these cancers. PAP (3′ phospho adenosine 5′ phosphate) is a known potent inhibitor of enzymatic sulfation. PAP exhibited Ki of 10μM with Gal3sulfo-T2 purified from colon cancer LS180 cells . Apparently, PAP may have use as metabolic inhibitor of these cancers.
The present study showed that α2-3 (O)ST (ST3Gal II) increased almost 100-fold in pancreatic tumorogenesis. We found earlier that this enzyme has reversible sialylation activity by converting 5′ CMP and also 5′ UMP to 5′ CMP-and 5′ UMP-NeuAc by utilizing the donor NeuAcα2-3Galβ1-3GalNAcα-units. Further, it was shown that 5′ UMP-NeuAc is an inactive sialyl donor for the sialylation of glycans by ST3Gal II, ST3Gal III and ST6Gal I [68]. 5′ UMP is apparently an efficient inhibitor of glycan sialylation process and has a potential of inhibiting pancreatic cancer progression.
We found in the present study neutrophils from diabetic retinopathy patients contained a high-level of glycosyltransferases involved in N- and O-glycan biosynthesis. In support of our finding it has been shown by others that increased involvement of the hexosamine pathway is a detrimental consequence of cell and tissue exposure to high glucose in diabetes. A transketolase inhibitor Benfotiamine was able to divert hexose metabolism to the pentose pathway and inhibit the development of diabetic retinopathy in animal models. KRAS variant controlled increase in hexosamine pathway for the growth of pancreatic tumor may as well be inhibited by specifically targeting pancreas with such metabolic inhibitors of hexosamine pathway for preventing pancreatic cancer invasion.
A recent report suggests that oral administration of mannose may be a safe and selective therapy in the treatment of cancer. It is apparent that oral mannose would affect the glycan patterns in pancreatic and other cancers. Some glycosyltransferases are shed in serum and β1-4 Gal-T attracted much attention as a biomarker for ovarian cancer. Thus, GlcNAc: β1-3/4GalNAc-T may have the potential to serve as pancreatic cancer serum biomarker that can be used for follow-up of cancer patients in oral mannose treatment.
We thank Dr. Harry Slocum, Dr. Karoly Toth and Ms. Nancy Reska for their invaluable technical assistance and cooperation in Tissue Procurement Resource (RPCI). We are indebted to Dr. P. Dandona for arranging fresh blood samples from control and diabetic retinopathy patients for the isolation of neutrophils.
The study was supported by NIH Grants CA35329, HL 103411, AI 56082 and Comprehensive Cancer Center Support Grant CA160561.
Citation: Chandrasekaran EV, DMarathe D, Neelamegham S, Lau JY, L Matta KL (2019) A high-level over-expression of N- and O- glycan glycosyltransferases in pancreatic tumors and diabetic neutrophils; An unique pathological situation in pancreatic cancer and diabetic retinopathy. J Glycobiol 8:138.
Received: 22-Apr-2019 Accepted: 20-May-2019 Published: 27-May-2019
Copyright: © 2019 Chandrasekaran EV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : NIH Grants CA35329, HL 103411, AI 56082 and Comprehensive Cancer Center Support Grant CA160561.