
Journal of Clinical and Cellular Immunology
Open Access
ISSN: 2155-9899

ISSN: 2155-9899
Research Article - (2021)Volume 12, Issue 6
Background and aim: Triple-negative breast cancer (TNBC) is defined as a group of breast carcinomas that are negative for expression of hormone receptors estrogen receptor (ER) or progesterone receptor (PR) and human epidermal growth factor receptor 2 (Her2). In India, several reports have suggested that TNBC incidence is higher and up to 31%. Histological features of triple-negative breast cancer are reported to be common with those of basallike subtype, comprising of high-grade invasive ductal carcinoma, no special type, invasive ductal carcinoma with a large central acellular zone, typical medullary carcinoma, and metaplastic carcinomas. In the present study, we aimed to correlate the pathological characteristics and evaluate IHC based expression of basal type biomarkers in triple negative breast carcinomas. Our study showed heterogeneity in histology, most of the TNBC cases in our study were IDC, NOS and there were a slightly higher percentage of atypical medullary cases as compared to other studies. Most of the cases were high grade based on modified NBR grading and majority of them turned out to be basal like on IHC. Distinctly found in our cases which had metastatic axillary lymph node was, basal immune phenotype unlike the case in other studies which demonstrated a higher proportion of lymph node negativity. As expected a high proportion of triple negative tumors showed a consistent basal cytokeratin expression (CK5/6-66%, CK14-72%, CK17-68%). EGFR and p53 positivity did not show statistical significance between basal and non-basal groups. Proliferation marker Ki67 was statistically significant in basal like groups. Gene expression profiling is the gold standard for TNBC molecular subtype classification, however, IHC is an accepted 'surrogate marker' for identifying and classifying the 'basal like group'. Our findings suggest that pathologic characteristics cannot be used to accurately classify triple-negative breast cancer into basal and non-basal groups.
Relevance to patients: DNA microarray based molecular profiling may not be accessible always in clinical settings, this study has emphasized that identification of basal like subtype of breast cancers can be done by doing easily available surrogate Immunohistochemical biomarkers on TNBC, and thus help to provide the patient with relevant target based therapeutic benefit.
Triple-negative breast cancer; Histology; Immunohistochemistry; Biomarkers
Worldwide 2,088,849 new cases of female breast cancer cases were reported in 2018, accounting for almost 1 in 4 cancer cases among women. In India, breast cancer is the most common cancer with 1,62,468 new cases diagnosed accounting and 87,090 deaths were reported in 2018 with a high incidence-to-mortality ratio (approximately 50%) according to GLOBOCAN 2018 [1]. Triplenegative breast cancer (TNBC) is defined as a group of breast carcinomas that are negative for expression of hormone receptors (ER, PR) and HER2. DNA microarray analysis have proved that triple-negative breast cancer are composed of the basal-like subtype and normal breast (or unclassified) subtype, the former being correlated with an aggressive clinical course. Histological features of triple-negative breast cancer are reported to be common with those of basal-like subtype, comprising of high-grade invasive ductal carcinoma, no special type, invasive ductal carcinoma with a large central acellular zone, typical medullary carcinoma, and metaplastic carcinomas. The basal-like subtype is characterized by the expression of myoepithelial/basal markers and molecular changes including p53 gene mutations, BRCA1 inactivation, and many chromosomal alterations [2-5]. TNBCs tend to present with more aggressive clinical features and have higher recurrence rates which make them a most aggressive subtype of breast cancer. In India, several reports have suggested that TNBC incidence is higher and up to 31% [6,7].
Gene expression analysis by DNA microarray technology is the gold-standard for identification of triple negative basal like breast cancers but it is not readily available in daily practice or for retrospective studies using formalin fixed, paraffin-embedded samples. In these situations protein expression characteristics bases on immunohistochemical staining (IHC) can be a useful surrogate of gene expression analysis. IHC is a simpler alternative and a more accessible technique. Immunohistochemical characterisation of basal-like cancer group dentified by gene expression profiling has shown that the majority of these tumors are triple negative and express markers of basal/myoepithelial cells such as basal cytokeratins (CK 5/6, 14 and 17), vimentin, epidermalgrowth factor receptor (EGFR) and have a high proliferation index [8-12].
To specifically sub classify TNBC, Lehmann et al. analysed the gene expression profile of 587 TNBC cases from 21 breast cancer databases and performed clustering analysis. Six subtypes were identified which may have therapeutic implications. The six subtypes identified by Lehman et al. are Basal like subtypes, BL1 and BL2, immunomodulatory (IM), Mesenchymal (M,Mmesenchymal stem like (MSL) and luminal androgen receptor (LAR) [13].
BL1 and BL2 subtypes have high expression of genes involved in cell cycle and cell division such as Aurora kinase and MYC and are highly proliferative as marked by high Ki-67 nuclear staining (BL1+BL2: 70% Vs. other subtypes: 42%). These results suggest that chemotherapies that target cell division and mitosis, such as taxanes, would be most applicable in this class. Indeed, BL1 and BL2 subtypes were associated with a significantly higher rate of CR (63%; p=0.042) with taxane-based therapies as compared to mesenchymal-like (31%) or luminal androgen receptor (14%) subtypes. A third subtype, immunomodulatory (IM), was found to be enriched in genes involved in immune processes. These include immune transduction pathways (NFKB, TNF, JAK), cytokine signalling such as IL-2 pathway, and antigen processing, among others. This subtype may represent medullary breast cancer, a subtype of TNBC that has a good prognosis, based on a similar expression profile reported in another study. Mesenchymal (M) and mesenchymal stem like (MSL) subtypes were characterized by expression of cell motility genes and proteins of the extracellular matrix. The MSL subtype displayed low expression of claudins 3, 4, and 7, consistent with the claudin-low subtype of breast cancer as previously discussed. MSL subtype also expressed genes involved in growth factor signalling such as EGFR and PDGFR pointing to possible therapeutic options in this subtype. The sixth subtype, luminal androgen receptor (LAR), was found to be enriched in genes involved in steroid synthesis the endocrine pathway despite being negative for ER and PR. This was replicated in the study by Lehmann et al. in that a distinct subtype of TNBC, LAR subtype, was identified that has high expressions of hormonal related genes [13].
Although the terms basal-like breast cancer and TNBC are often used interchangeably, they are not synonymous. TNBC refers to the immunophenotype of the breast cancer which is immunologically negative to ER, PR, and HER2. These immunological studies are done on formalin-fixed and paraffin-embedded tumor sections. Basal-like breast cancer refers to the molecular phenotype of the tumor that has been defined by cDNA microarrays. Of these TNBCs, about 75% of them are of basal-like type [14]. In a study, Bertucciet et al. reported that 37 (23%) tumours defined as basal-like by GEP showed a non-TN phenotype, while 49 (29%) tumours with a TN phenotype were defined as non-basal by gene expression profiling [8]. Emad et al. [15] have demonstrated that the expression of basal markers (i.e. basal cytokeratins and EGFR) identifies a clinically significant subgroup within the triple-negative group. Furthermore, expression of basal cytokeratins and/or EGFR, regardless of the expression of ER or PR status, identifies a subgroup of cancers which display a particularly poor prognosis; emphasising the prognostic value of these basal markers expression irrespective of the hormone receptors status. Most (68%–86%) basal-like tumors are invasive ductal carcinomas of no special type, but occasionally, the carcinoma is tubular mixed. Basal-like carcinomas are usually of high histologic grade; 75% to 100% are grade 3. Other important histologic features include pushing, noninfiltrative borders of invasion; large zones of geographic or comedo-type necrosis; stromal lymphocytic infiltrates; scant stromal content; lack of tubule formations; marked cellular pleomorphism; high nuclear–cytoplasmic ratios; vesicular chromatin; prominent nucleoli; high mitotic indices; and frequent apoptotic cells [15].
Basal cytokeratin (CK), EGFR, Ki67 and p53 expression in TNBC
Because basal CKs expression is one of the main characteristic features of basal-like tumors, most but not all IHC studies have used them to defined basal-like tumors (Table1). Additional markers used to define basal-like tumors, although associated with basal CK expression, so far have not helped improve the identification of cases with differing outcome compared with those identified using basal CKs alone. These additional markers, if used to define cases of basal-like cancer, would reduce considerably the proportion of cases allocated to this poor prognosis type of breast cancer. Therefore, a suitable, pragmatic solution would be to use basal CKs expression to define basal-like tumors regardless of the expression of other markers [16].
| Study | Definition |
|---|---|
| Nielsen et al. | ER/HER-2–and CK5/6 and/or EGFR |
| Garcia et al. | CK5/6, caveolin 1, CAIX, p63 or CD117 |
| Rakha et al. | CK5/6 and/or CK14 |
| Jumppanen et al. | CK5/14 |
| Rakha and Ellis. | C5/6, CK14, CK17 and EGFR of which at-least 2 should be positive to be termed as Basal-like breast cancer |
Table 1: Definition of basal like cancers in different studies.
HER1 (EGFR) is a receptor tyrosine kinase, that belongs to the HER family of transmembrane receptors. HER1 expression is higher (up to 80%) in TNBC and metaplastic carcinoma (mostly basal-like), where it possibly substitutes ineffective, but otherwise major proliferation/survival pathways of breast cancer induced by expression and activation of HER2, ER and PR proteins. Currently, however, HER1 gene status is not used in clinical practice to guide therapy in breast cancer [17].
Ki67 is a labile, nonhistone nuclear protein that is tightly linked to the cell cycle. The proliferation marker Ki-67 has repeatedly been confirmed as an independent predictive and prognostic factor in early breast cancer. TNBC is associated with a higher expression of Ki-67 than non-TNBC [18]. p53 protein is involved in major cell cycle control pathways frequently targeted in human tumorigenesis. p53 functions to eliminate and inhibit the proliferation of abnormal cells, thereby preventing neoplastic development. Mostly all human cancers show dysregulation of p53 pathways. Prognostic value of p53 mutations in breast cancer has been shown in various studies; however their role as expression in TNBC has not been fully understood [19,20].
In the present study, we aimed to correlate the pathological characteristics and evaluate expression of basal type biomarkers in triple negative breast carcinomas.
This was a retrospective study conducted at Triesta science research laboratory of HealthCare Global Enterprises Ltd., Bangalore and was approved by Institutional Ethics Committee.
In this study, female patients diagnosed with triple negative invasive breast carcinoma, at this Institute between January 2009 to December 2011 were included. Histopathological and Immunohistochemical studies were done on formalin fixed paraffin embedded tissue blocks. Formalin fixed paraffin embedded tissue blocks of all the patients enrolled in this study were collected and processed for histopathological details and reviewed for prognostic features and triple negative morphology.
Histopathological analysis
The triple negative invasive breast tumors were typed using the World Health Organization (WHO) breast tumours classification [21]. Medullary and atypical medullary carcinoma was defined according to the scoring system of Ridolfi et al. [22]. The tumor grade was scored using the Modified system of Bloom and Richardson by assessing mitotic rate, tubular differentiation, and nuclear pleomorphism [23]. Mitotic counts were performed using the 40X objective on an Olympus CX51 microscope. The presence or absence of In Situ carcinoma component was noted. The intraductal components were classified according to the classification system of ductal carcinoma In Situ (DCIS) [24]. Tumor block was examined for the various morphological parameters such as mentioned in Table 2.
| Morphological features |
|---|
| Appearance of tumor margin, pushing or infiltrative |
| Geographic necrosis( mild, moderate or severe) |
| Prominent central sclerosis |
| Type of nuclear chromatin vesicular or coarse |
| The presence of lymphoid stromal infiltrate (mild, moderate to marked) |
| Presence or absence of nucleoli |
| Desmoplasia (mild, moderate or severe) |
| Squamoid or spindle cell changes |
| Presence of apocrine differentiation |
| Metaplastic differentiation |
Table 2: List of various morphological parameters in TNBC studied in the tissue sections.
Immunohistochemical analysis
Immunohistochemical markers were studied using a one-step polymer-HRP detection system used [25]. A detection system using a non-biotin polymeric technology that makes use of only one major component a polymer–HRP reagent. This reagent consists of both secondary immunoglobulin molecules and horseradish peroxidase molecules linked to a common dextran polymer backbone, thus eliminating the need for sequential application of link antibody and peroxidase-conjugated antibody. Ready to use primary antibodies used for IHC studies were viz., CK5/6 (D5/16 D4, Dako), CK14 (LL002, Biogenex), CK17 (E3, Dako), p53 (DO- 7, Dako), Ki67 (BGX297, Biogenex) and EGFR (EP38Y, Biogenex). Tissues sectioned, mounted on charge slides and dewaxed. Further slides were treated with an antigen retrieval solution [EDTA buffer (1Mm, pH 8)] if required, blocked with a proteinaceous blocking solution (3% BSA) and then incubated with the primary antibody. The bound primary antibody was detected by the addition of secondary antibody conjugated with horseradish peroxidise polymer and DAB substrate. When adequate colour developed, the slides were washed in water to stop the reaction, counterstained with Harris Haematoxylin, and covered with a mounting medium (DPX).
Interpretation of IHC results
For CK5/6, CK14 and CK17 markers, any cytoplasmic expression in definite neoplastic cells or tissue was considered as positive result [26]. For p53, positive staining was defined as positive nuclear staining of tumour cells. The cutoff point of 10% was taken for interpretation. If more than 10% of tumour cells having moderate to strong nuclear staining was seen, the test was interpreted as positive. If ≤ 10% of tumour cells had moderate to strong staining, the test was interpreted as negative [27].
Ki-67 positive staining was defined as positive nuclear staining of tumour cells. The percentage of positive nuclei expressing Ki-67 was determined by counting 1000 cells/slide. The scoring is defined as follows: less than 10%=low proliferative activity, 10-40%=moderate proliferative activity and more than 40%=high proliferative activity [28].
Epidermal Growth Factor Receptor (EGFR) protein expression by IHC was considered positive only if membrane staining was observed in invasive malignant cells. EGFR staining was further defined as follows; 0 (No membrane staining), 1+ (faint, partial membrane staining), 2+ (weak, complete membrane staining in >10% of invasive cancer cells) and 3+ (Intense, complete membrane staining in >10% of invasive cancer cells). Tumors with 0 and 1+ immunoreactivity were interpreted as negative for EGFR over expression. Only tumours with 2+ and 3+ immunoreactivity in >10% of invasive cancer cells were interpreted as positive for EGFR over expression [26].
All cases were further sub classified as basal, nonbasal and uncertain category based on immunohistochemistry using positivity for any 2 of the basal markers (CK5/6, CK14, CK17, EGFR). Uncertain category was used when only 1 of the basal markers was positive.
Patient data collection
Demographic and clinical details were collected by reviewing the medical records of the patients. Details of age, sex, location of the tumour with respect to the side (right or left or bilateral) and quadrant of breast involved were noted. Relevant personal history and family history were also noted.
Statistical analysis
Data was analyzed using SPSS VERSION 18 for windows. We used a test for proportions non parametric Chi Square/correlation analysis to compare differences in proportions of categorical variables of interest between Basal and Non basal groups. Statistical values of p<0.05 were considered as significant.
50 female patients diagnosed with triple negative invasive breast carcinoma, who fulfilled the selection criteria were included in this study. A morphological and immunohistochemical study was done. These cases were further sub classified as basal or non-basal based on presence or absence of high molecular weight cytokeratin (CK5/6, CK14, CK17) and EGFR respectively. A few cases showed expression of either EGFR or high molecular weight CK but not both and hence were categorized as “Uncertain”.
Basal like Breast Cancers: 43 of the 50 triple negative cases showed a typical basal like (86%) immunophenotype.
Non Basal: 2 of the 50 triple negative cases (4%) did not show a typical basal IHC profile
Uncertain: 5 of 50 triple negative cases (10%) on IHC showed an inconclusive immunoprofile.
Age Distribution: Table 3 shows age distribution pattern of molecular subtypes among TNBC cases in our study. It has been observed that basal tumours (n=24) occur more frequently in the peri and pre-menopausal age group (i.e. <50 years) when compared to the non-basal group (n=2).
| Age distribution | Basal (n=cases) |
Non basal (n=cases) | Uncertain (n=cases) |
|---|---|---|---|
| ≤ 40 yrs | 9 | 0 | 1 |
| 41-50 yrs | 15 | 2 | 1 |
| 51-60 yrs | 9 | 0 | 0 |
| >60 yrs | 10 | 0 | 3 |
Table 3: Age distribution of molecular subtypes among TNBC cases.
Distribution of morphological subtypes of TNBC
Our study had heterogeneity in histology. Of the 50 TNBC cases 27 were infiltrating ductal carcinoma (IDC) NOS type, 14 cases were atypical medullary, 3 cases were invasive lobular carcinomas, 1 case was mixed ductal and lobular and 2 cases were medullary carcinoma and 3 were metaplastic carcinoma. Figure 1 shows distribution of morphological types in TNBC cases in our study.
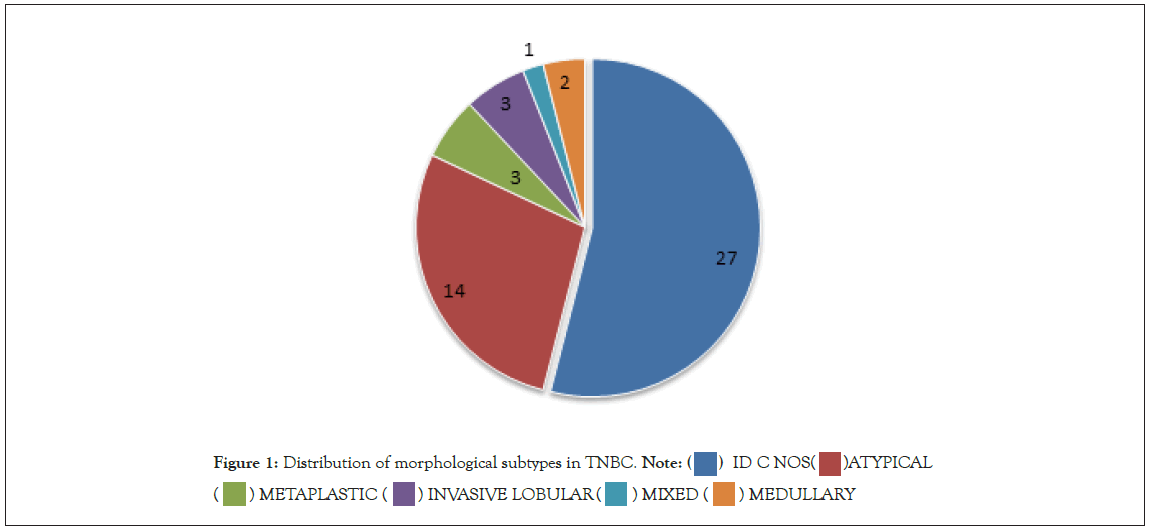
Figure 1: Distribution of morphological subtypes in TNBC. Note: (  ) ID C NOS(
) ID C NOS( )ATYPICAL
(
)ATYPICAL
( ) METAPLASTIC (
) METAPLASTIC ( ) INVASIVE LOBULAR (
) INVASIVE LOBULAR ( ) MIXED (
) MIXED ( ) MEDULLARY
) MEDULLARY
Correlation of morphological features in TNBC cases in basal and non-basal groups
Table 4 summarizes correlation of various morphological features in our TNBC study cases in basal and non-basal groups.
| Basal Type Morphology | Basal based on IHC (%) | Non basal based on IHC (%) | Uncertain based on IHC (%) | X2 | p-value |
|---|---|---|---|---|---|
| Tumor border Pushing Infiltrating |
21 (87-5%) 22 (84-6%) |
1 (4.2%) 1 (3.8%) |
2 (8.3%) 3 (11.5%) |
0.143 | 0.931 |
| Nuclear atypia Moderate atypia Severe atypia |
1 (33.3%) 42 (89.1%) |
1 (33.3%) 1 (2.2%) |
1 (33.3%) 4 (8.7%) |
9.398 | 0.009 |
| Geographic necrosis Absent Mild Moderate Severe |
16 (84.2%) 9 (100%) 9 (90%) 9 (75%) |
1 (5.3%) 0 (0%) 0 (0%) 1 (8.3%) |
2 (10.5%) 0 (0%) 1 (10%) 2 (16.7%) |
3.237 | 0.779 |
| Apocrine differentiation Present Absent |
2 (50%) 41 (89.1%) |
0 (0%) 2 (4.3%) |
2 (50%) 3 (6.5%) |
7.786 | 0.20 |
| Mitotic figures 0-7/10 HPF 8-14/10 HPF ≥ 15/10 HPF |
1 (50%) 13 (100%) 29 (82.9%) |
0 (0%) 0(0%) 2 (5.7%) |
1 (50%) 0 (0%) 4 (11.4%) |
6.066 | 0.194 |
| Nucleoli Present Absent |
40 (85.1%) 3 (100%) |
2 (4.3%) 0 (0%) |
0 (0%) 5 (10.6%) |
0.520 | 0.771 |
| Lymphocyte response Absent Mild Moderate Dense |
1 (100%) 4 (80%) 20 (90.9%) 18 (81.8%) |
0 (0%) 0 (0%) 1 (4.5%) 1 (4.5%) |
0 (0%) 1 (20%) 1 (4.5%) 3 (13.6%) |
1.968 | 0.923 |
| Nuclear chromatin Vesicular Coarse |
36 (83.7%) 7 (100%) |
2 (4.7%) 0 (0%) |
5 (11.5%) 0 (0%) |
1.325 | 0.516 |
| Central sclerosis Present Absent |
37 (90.2%) 6 (66.7%) |
2 (4.9%) 0 (0%) |
2 (4.9%) 3 (33.3%) |
6.892 | 0.32 |
| Metaplastic differentiation Present Absent |
4 (100%) 39 (84.8%) |
0 (0%) 2 (4.3%) |
0 (0%) 5 (10.9%) |
0.708 | 0.702 |
Table 4: Correlation of morphological features in TNBC cases in basal and non-basal groups.
Lymphovascular Invasion (LVI)
Of the 50 cases studied, 30 cases (60%) showed LVI and in the remaining 20 cases (40%) it was not observed. Further categorization with IHC showed that of the 30 cases with LVI 25 were basal, 1 was non basal and 4 were of the uncertain group. There was no statistical significance with basal group of tumours. Representative image of LVI is shown in Figure 2A.
Figure 2: DRepresentative images of morphological features seen in our TNBC cases A. Lymphovascular invasion (H&E X 400), B. Central Sclerotic zone (H&E X 100), C. Severe nuclear atypia (H&E x 1000), D. High Mitotic figure, E. Dense lymphocyte response (H&E X 400) and F. Apocrine differentiation (H&E X 400).
Lymph node status distribution
Of the 50 TNBC cases studied, 24 cases had axillary lymph node metastases and 25 cases had no lymph node metastases, for 1 case axillary dissection was not done. Further categorization with IHC showed that among these 24 cases with nodal metastases 19 cases showed a basal immunophenotype, 1 was non basal and 4 were of the uncertain group. In the 25 cases without nodal metastases 23 were basal, 1 was non basal and 1 was uncertain. Apical lymph nodal metastasis was seen in only 2 cases of which 1 was basal and other was of non-basal group.
Central sclerotic zone
Of the 50 TNBC studied, central fibrotic zone was seen in 41 cases (82%) and was not observed in 9 (18%) cases. Further categorization with IHC showed that of the 41 cases with central fibrotic zone, 37 were found to be BLC, 2 were uncertain and 2 cases were non basal. Representative image of Central Sclerotic zone is shown in Figure 2B.
Correlation of nuclear grade in TNBC basal and nonbasal groups
Of the 50 TNBC cases 47 were having severe nuclear atypia (grade 3) and 3 had (6%) moderate nuclear atypia (grade 2). Further categorization with IHC revealed that 42 of the 47 cases with severe nuclear atypia were basal like on IHC and 1 was non basal and 4 were uncertain. Of the 3 cases with moderate atypia 1 case was basal, 1 was non basal and 1 was uncertain (Figure 3).
Figure 3: Corrrelation of nuclear grade in TNBC basal and non-basal groups.
Correlation of mitotic figure in TNBC basal and nonbasal groups
Of the 50 TNBC cases studied 35 (70%) cases had score 3 for mitotic figures, 13 (26%) cases had score 2 and 2 (4%) cases had score 1 MF. Further categorization with IHC showed that. Of the 35 cases with score 3 MF 29 were basal like, 2 were non basal and 4 were uncertain based on IHC, All 13 cases with score 2 were basal like on IHC. Of the 2 cases with score 1 MF, one case was basal and the other was uncertain on IHC (Figure 4).
Figure 4: Correlation of mitotic figures in TNBC basal and non-basal groups.
Lymphocyte response
Of the 50 TNBC cases studied, lymphocytic infiltration was seen in 49 cases. Further categorization with IHC showed that of the 49 cases, 44 had dense to moderate lymphocytic infiltration, out of which 38 cases were BLC based on IHC, 4 cases were uncertain, 2 were non basal. 4 of 5 cases with mild lymphocyte response had basal marker positivity while 1 case was uncertain (Figure 5).
Figure 5: Correlation of lymphocyte response in TNBC basal and non-basal groups.
Apocrine differentiation
Among the 50 TNBC cases 4 had apocrine differentiation and in the remaining 46 cases this feature was not observed (Figure 2F). Further categorization with IHC showed that 3 of 4 cases which had apocrine differentiation were BLC based on IHC and 1 was uncertain. Among the 46 cases with no observable apocrine differentiation 41 were BLC, 2 were non basal and 3 were uncertain.
Metaplastic differntiation
Of the 50 TNBC cases studied, metaplastic differentiation was observed in 4 (8%) cases and was not observed in 46 (92%)cases. All cases which showed metaplastic differentiation had basal immunohistochemistry proving their basal like origin (Figure 6A and B).
Figure 6: Representative images of morphological features A. Chondroid metaplasia (H&E X 400), B. Squamoid metaplasia(H&E X 400), C. Intensity of desmoplasia (H&E X 400).
Desmoplasia
All the 50 TNBC cases had varying intensity of desmoplasia. 4 cases had severe desmoplasia, 36 cases had moderate desmoplasia and 10 cases had only mild desmoplasia Intensity of desmoplasia is shown in Figure 6C. Further categorization with IHC showed that. All 4 cases which had severe desmoplasia showed basal phenotype. 7 of 10 cases which had mild desmoplasia were basal like and 3 cases where uncertain. 32 of 36 cases with moderate desmoplasia were basal like and 4 were of the uncertain group.
Correlation of immunohistochemical expression of CK5/6, CK14, CK17, EGFR, p53 and Ki67 biomarkers in our TNBC cases in both basal and non-basal groups
Table 5 summarizes correlation of expression of biomarkers in TNBC cases in basal and non-basal groups. Representative immunostaining pattern of different biomarkers is shown in Figure 7.
| IHC marker | Basal based on IHC | Non basal based on IHC | Uncertain based on IHC | X2 | p-value |
|---|---|---|---|---|---|
| CK5/6 Positive Negative |
32 (97%) 11 (64.7%) |
0 (0%) 2 (11.8%) |
1 (3%) 4 (23.5%) |
9.995 | 0.007 |
| CK14 Positive Negative |
36 (100%) 7 (50%) |
0 (0%) 2 (14.3%) |
0 (0%) 5 (35.7%) |
20.93 | 0.000 |
| CK17 Positive Negative |
34 (100%) 9 (56.2%) |
0 (0%) 2 (12.5%) |
0 (0%) 5 (31.2%) |
17.29 | 0.000 |
| EGFR Positive Negative |
32 (74.4%) 11 (25.6%) |
1 (50%) 1 (50%) |
3 (60%) 2 (40%) |
0.962 | 0.618 |
| p53 Positive Negative |
30 (69.8%) 13 (30.2%) |
1 (50%) 1 (50%) |
2 (40%) 3 (60%) |
2.006 | 0.367 |
| Ki67 <10% 10-40% >40% |
2 (4.7%) 5 (11.6) 36 (83.7%) |
0 (0%) 2 (100%) 0 (0%) |
1 (20%) 2 (40%) 2 (40%) |
14.739 | 0.006 |
Table 5: Correlation of IHC based expression of biomarkers in TNBC cases in basal and non-basal groups
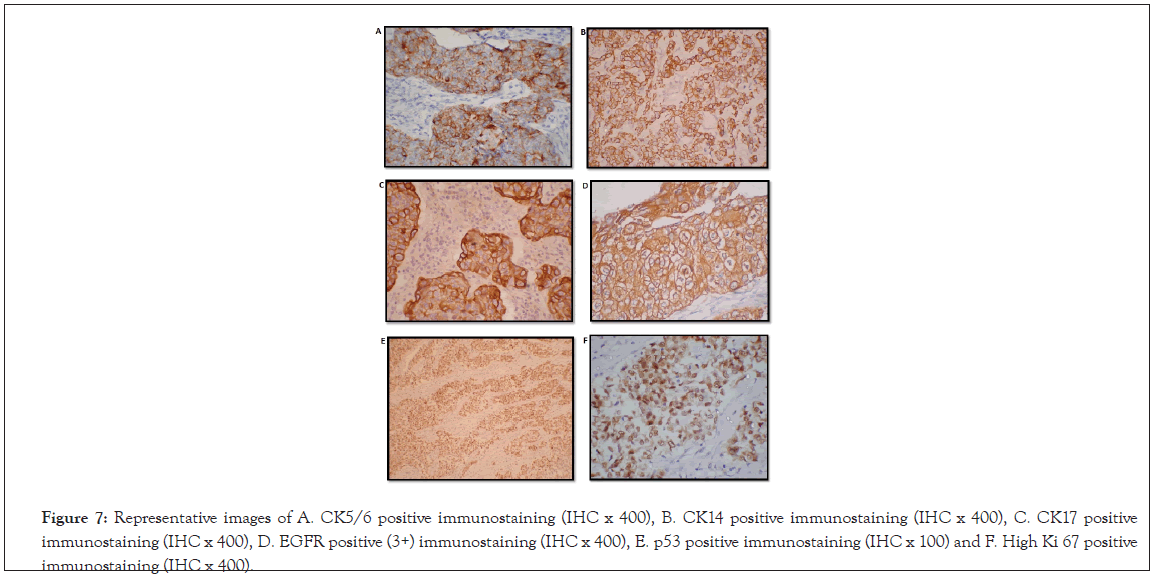
Figure 7: Representative images of A. CK5/6 positive immunostaining (IHC x 400), B. CK14 positive immunostaining (IHC x 400), C. CK17 positive immunostaining (IHC x 400), D. EGFR positive (3+) immunostaining (IHC x 400), E. p53 positive immunostaining (IHC x 100) and F. High Ki 67 positive immunostaining (IHC x 400).
Among all the markers CK5/6, CK14, CK17 and Ki67 were significantly highly expressed in basal type as compared to nonbasal and uncertain.
CK5/6, CK14 and CK17 immunohistochemical expression
33 cases were positive for CK5/6 immune expression (Figure 7A). IHC showed that 32 of the above mentioned 33 cases showed positivity for more than 1 basal marker (excluding CK5/6) and thus were grouped under ‘basal like’. The remaining case with CK5/6 positivity did not show positivity for any of the other basal markers like CK17 etc and was hence was grouped in the ‘uncertain’ category.
CK14 positivity (Figure 7B), was seen in 36 cases (72%), all of which further subcategorized as basal based on the selection criteria mentioned above. CK17 immunopositivity (Figure 7C) was seen in 34 cases. All 34 cases were further subcategorized as belonging to the basal group showing positivity for more than 1 basal marker.
EGFR, p53 and Ki67 immunohistochemical expression
EGFR was positive (Figure 7D) in 36 cases (72%) and was negative in 14 cases (28%). Among the 36 EGFR positive cases, 32 were categorized as basal and 4 were uncertain as no other basal marker was positive. Among the 14 EGFR negative cases, 11 were of the basal phenotype basal, 2 were uncertain and only 1 was non basal.
p53 immunopositivity (Figure 7E) was seen in 33 (66%) cases showed p53 positivity and 17 cases (34%) were negative for p53. Further, 30 of the 33 positive cases showed a basal phenotype, 1 was non basal and 2 were uncertain. Of the 17 cases which were negative for p53, 13 were basal, 1 was non basal and 3 were uncertain.
Ki67 (Figure 7F) expression was seen high in 38 cases (76%), 9 (18%) cases had medium and 3(6%) had low Ki67 expression. 36 cases among the high Ki 67 were basal and only 2 were uncertain. Among the 9 cases with medium Ki 67, 5 were basal, 2 were non basal and 2 were uncertain. Among 3 cases with low Ki 67, 2 were basal and 1 was uncertain (Figure 8).
Figure 8: Correlation of Ki 67 expression in TNBC basal and non-basal groups.
In this present study, we aimed to pathologically illustrate the morphology of triple-negative breast cancer in an institutional setting (a tertiary care oncology institute). We also subjected these tumours to a broad panel of biomarkers, including those of a basal nature, to better comprehend the relationship between triple negative and basal-like breast cancers in our women. In our study, the group of triple-negative cancers was heterogeneous. Most of the tumors were found to be high grade IDC NOS, or atypical medullary carcinoma. We studied the various morphological features of TNBC more commonly seen in basal like breast cancers like-pushing tumour border, geographic necrosis, central sclerosis, prominence of nucleoli, vesicular nuclear chromatin, dense lymphocyte response, apocrine and metaplastic differentiation.
Using the basal cytokeratin expression (CK5/6,CK14,CK17) and EGFR these triple negative cases were subdivided into basal and non-basal groups. Furthermore the clinicopathologic and biological differences between TN tumors that express basal markers [CK5/6, CK17, CK14, and EGFR] and those that are negative for these basal markers using a series of 50 invasive breast cancers with 2 proliferation markers(Ki67, p53) was highlighted in this study.
Despite the great interest in basal-like cancers, there is still no internationally accepted definition of these tumours. From a scientific point of view, microarray based expression profiling analysis remains the “golden standard” for identification of basallike breast cancers. This technology is unlikely to be introduced in daily routine diagnostic practice in the foreseeable future, and results of microarray based expression profiling using RNA extracted from formalin-fixed archival samples are suboptimal. Several attempts to define an immunohistochemical surrogate for basal-like cancers have been described. The best example to date is the panel proposed by Nielsen et al. [29] where basal-like cancers are defined as those lacking ER and HER2 expression and expressing CK5/6 and/or EGFR. This panel has a specificity of 100% and a sensitivity of 76% for the identification of basal like cancers [30].
In this study most of the triple negative patients, 56% were ≤ 50 yrs of age and 44% were >50 yrs of age. A review of world literature on epidemiology shows that, mean standard age for TNBC to be 49 to 51 yrs [31,32]. Such variations may be because our study sample size is restricted to 50 cases. Also, we are limited by a paucity of publications with accurate epidemiological assessment of south Indian population based on age. Most of the TNBC cases in our study were IDC, NOS (54%) and 28% cases were atypical medullary, 6% cases were invasive lobular, 8% cases were metaplastic carcinomas, 2% were mixed ductal and lobular and 4% were medullary. Most of the literatures reviewed [30,32,33] showed IDC, NOS as the predominant histological subtype seen in TNBC with proportions ranging from 72% to 96%. This differences in proportion when comparing our study to world literature can be attributed to the small sample size (n=50)
The proportion of medullary subtype have shown much fluctuation, one of the study showed 2% [34] another showed 3.7% [33] and still others [30] had 22%. Metaplastic carcinomas were between 1% to 3% [33,34]. ILC ranged from 1 to 2% [34]. The proportion of these minor subtypes was found to be more or less similar to that found in our study. 96% of TNBC cases in our study were high grade compared to other studies that have shown prevalence of high grades in TNBCs to range from (58% to 85%) [32-34]. However 4% grade II cases seen in our study was lesser than earlier studies 6% to 21% [32,34,35]. This could probably be due to the fact that other studies were done in western population unlike ours (Indian population) which could have accounted for the variation in TNBCs presentation.
On application of IHC, a significant proportion of our high grade tumours (82%) were of the ‘basal group’ and is in concordance with other major study groups ,who have stated 75 to 100% cases of high grade tumors to be basal like [2,37]. In our study 36% cases were having In Situ carcinomas and in the remaining 64% cases In Situ carcinoma were not observed. Of the studies reviewed [10,34,36] have shown a wide range of cases with In Situ carcinomas to be 41% to 81%. Such discordance with our study may be because of small sample size. Most of the cases in our study showed lymphovascular invasion (60%) and in the rest 40% this feature was not observed. Two studies showed LVI were between 13% to 37% [33,34].
Our study showed 98% TNBC cases with lymphocytic infiltration, which is similar to other studies which found around 87%- 100% lymphocytic infiltration [10,34]. We got 85% cases with lymphocytic infiltration to be basal like based on IHC while one study [2] showed only 56% of basal like cases to have lymphocyte response. That study had studied only 23 basal like cases and so there number was less. Mitotic figures (MF) in our TNBC cases were, 4% with score 1, 26% with score 2 and those with score 3 were 70%. These figures are similar to the study [34] done on TNBC which had score 3 MF in 69% cases, score 2 MF in 21% cases and score 1 MF in 9 % cases. We had 8% of the TNBC cases with metaplastic features and all of them were basal. On reviewing other studies [10,34] the proportion showed 1 to 3% of the TNBC cases with metaplastic features. This could be because our hospital is a tertiary Centre and we get many referral cases from different institutes all over India.
CK5/6 immunopositivity was about 66% in our study, different studies [10,26,37] have found varying percentages of positivity ranging from 31% to 76%.Our statistics is towards the higher side of this range. P value was significant (p=0.007). In our study we got 72% of the TNBC cases showing positivity for CK14, P value was significant (p=0.007), while other studies [34,37] have shown 48% to 54% TNBC cases to be CK14 positive. CK17 expression was seen in about 68% of our TNBC cases, P value was significant (p=0.007). Other studies [34] have found the percentage of CK17 positivity to be around 50% in TNBC cases. EGFR positivity was seen in 72% of cases in our study. On reviewing other literatures [10,26,30,31] have reported varying percentages of EGFR positivity in TNBC cases ranging from 26% to 74%. P value was not significant. p53 expression was positive in 66% of our study cases, which is similar to the findings reported from other studies in the range of 39% to 61% [2,38]. We also found that 90% of these cases with p53 positivity were basal like on IHC, P value was not significant. This is also similar to other studies which have found 82% of basal like cancers showing p53 positivity. In our study 76% of the TNBC cases were having high expression of Ki67. Other studies [38,39] have found Ki67 expression in TNBC ranging from 58% to 73%. TNBC cases which were classified as basal based on IHC with high Ki67 were 94%, P value was significant (<0.005). We found 86% TNBC cases in our study which were basal like based on IHC, this is similar to that shown in other studies varying from 75% to 84% [30,34,40] based on basal cytokeratin expression and EGFR.
The following conclusions were drawn from a three year study (prospective and retrospective) on a sample size of 50 Triple negative breast cancer cases diagnosed with invasive breast carcinoma. Our study had heterogeneity in histology, most of the TNBC cases in our study were IDC, NOS and there was a slightly higher percentage of atypical medullary cases as compared to other studies. Most of the cases were high grade based on modified NBR grading and majority of them turned out to be basal like on IHC. Distinctly found in our cases which had metastatic axillary lymph node, was basal immune phenotype unlike the case in other studies which demonstrated a higher proportion of lymph node negativity. Lymphocytic infiltration was seen in majority of our cases demonstrating a basal immune profile. This is similar to other triple negative studies. Metaplastic differentiation and apocrine differentiation showed statistical significance with basal like group. As expected a high proportion of Triple negative tumors showed a consistent basal cytokeratin expression (CK5/6-66%, CK14-72%, CK17-68%). Each category demonstrated statistical significance (p value as demonstrated). EGFR and p53 positivity did not show statistical significance between basal and non-basal groups. Proliferation marker Ki67 was statistically significant in basal like groups.
These findings suggest that pathologic characteristics cannot be used to accurately classify triple-negative breast cancer into basal and non-basal groups. Though gene expression profiling is the gold standard, IHC is an accepted 'surrogate marker' for identifying and accurately classifying the 'basal like group'. Due to a limited sample size in this study, the statistical significance or P value of some of the parameters studied could not be well delineated. Also morphological characteristics and 'basal like characteristics' can be better evaluated on a larger sample size study.
The authors declare that they have no conflict of interest
Citation: Maheshwari P, Kumar R, Hazarika D, Naik R (2021) A Correlation of Pathological Characteristics and Immuno Histo Chemistry (IHC) Based Biomarker Expression in Triple Negative Breast Carcinomas. J Clin Cell Immunol. 12:631.
Received: 11-Oct-2021 Accepted: 25-Oct-2021 Published: 01-Nov-2021 , DOI: 10.35248/2155-9899.21.12.631
Copyright: © Maheshwari P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.