Journal of Applied Pharmacy
Open Access
ISSN: 1920-4159
ISSN: 1920-4159
Review Article - (2023)Volume 15, Issue 2
This review on Floating Drug Delivery Systems (FDDS) was written with the intention of gathering the most recent research with a particular focus on the main mechanism of flotation to induce stomach retention. The most current advancements in FDDS are reviewed in depth, along with the physiological factors and formulation factors impacting stomach retention, design strategies for single-unit and multiple-unit floating systems and their classification and formulation characteristics. The advantages and future prospects for oral controlled drug delivery of the present technological breakthroughs of FDDS, including patented delivery methods and marketed products, are described in this review.
Floating drug delivery systems; GIT physiology; Approaches to design floating dosage form; Floating polymers; Marketed floating products
The primary goal of the oral controlled Drug Delivery System (DDS) should be to enhance and increase predictability of medication bioavailability. The emptying process may take anywhere from a few minutes to 12 hours [1]. Because it is simple to administer, inexpensive and offers a large range of formulation options, the oral route is typically regarded as the best method for drug delivery in single dose systems. Systems for oral medication delivery that deliver a single dose of the drug directly to the target site and maintain the desired drug concentration for a prolonged period of time are ideal. In order to improve patient compliance and reduce administration frequency, extensive research has been done to create single dose controlled or sustained drug release systems. When opposed to medications that are just locally active in the stomach, including antacids and antibiotics, Gastro Retentive Drug Delivery Systems (GRDDS) exhibit an additional benefit of increased bioavailability. For the treatment of gastrointestinal illnesses like gastro esophageal reflux, the most significant benefit of GRDDS is site-specific medication delivery. Improved bioavailability, a decrease in dosage and a reduction in gastrointestinal adverse effects are all anticipated from slow but complete drug release in the stomach. This increases patient compliance while also extending the time between medicine doses.
The literature has described a number of methods for creating dose forms that can be kept in the stomach:
• Low density systems
• High density systems
• Swelling and expanding systems
• Ultra-porous hydro gels
• Hydro-dynamically balanced systems
• Gas generating systems
• Raft forming systems
• Floating systems
• Ion exchange resins
Dose forms that stay in the stomach longer than standard dosage forms, the capacity to extend and control the emptying time is a major asset [2]. Gastric emptying of dosage forms is a highly variable process. Designing controlled release systems for greater absorption and increased bioavailability presents a number of challenges. The difficulty to limit the dose form in the desired region of the gastrointestinal tract is one of these challenges. The process of drug absorption from the digestive system is intricate and influenced by a variety of factors. It is well accepted that the length of time a medicine spends in contact with the small intestinal mucosa determines how much of the gastrointestinal tract it will absorb. Because of this, the small intestinal transit time is a crucial factor for medications that are only partially absorbed. Basic human physiology is discussed, including information on gastric emptying, motility patterns and physiological and formulation factors that affect cosmic emptying. For medications that are less soluble in a high pH environment, prolonged stomach retention increases bioavailability, lowers drug waste and enhances solubility. It can be used to administer medications locally to the stomach and nearby small intestines. Gastro retention makes novel goods with new treatment options and significant patient advantages more readily available [3].
The simple administration and handling of oral dose forms contributes to the high level of patient compliance. Although oral controlled drug delivery systems have made significant strides over the past two decades, they have had limited efficacy when used with medications that have a poor absorption window throughout the GIT (Gastro Intestinal Tract). One of the biggest difficulties in developing an oral controlled medication delivery system is changing the GI transit time. Drugs with a brief half-life are swiftly removed from the bloodstream. In order to get around these issues and release the medication such that it can maintain its plasma concentration for a longer amount of time, several oral controlled delivery methods have been developed [4]. For medications that are less soluble in a high pH environment, prolonged stomach retention increases bioavailability, lowers drug waste, and enhances solubility. It can be used to administer medications locally to the stomach and nearby small intestines. Gastric retention makes new drugs with novel therapeutic potential and significant patient benefits more readily available. By using the mechanisms of much adhesion, flotation, sedimentation, expansion, changed shape systems or the concurrent administration of pharmacological drugs that delay gastric emptying, solid dose forms can be retained in the stomach under regulated conditions [5].
GIT physiology
The term "human gastrointestinal tract" usually refers to the stomach and intestines, but it can also apply to the entire system, including the mouth and the anus. (The word "digestive system" is more inclusive and includes additional structures, such as the auxiliary organs of digestion.) The Gastrointestinal (GI) tract measures 5 meters (20 feet) in length in an adult male person (Figure 1).
Figure 1: Human gastrointestinal tract.
Upper gastrointestinal tract
The oesophagus, stomach and duodenum make comprise the upper digestive system. Muscle tone, and consists of the upper and lower GI tracts. Some sources additionally mention the mouth cavity and pharynx. Foregut, midgut and hindgut are additional classifications for the tract that reflect the embryological origin of each section. To help control the digesting process, the GI tract releases hormones. Gastrin, secretin, cholecystokinin and grehlin are just a few of the hormones that are mediated by either intra or auto crine pathways, showing that the cells that release these hormones have remained relatively constant over the course of evolution. The precise line between "higher" and "lower" can differ. The duodenum may appear to be a single organ upon gross dissection; however it is frequently divided into two portions based on function, vascular supply, or embryology (Figure 2).
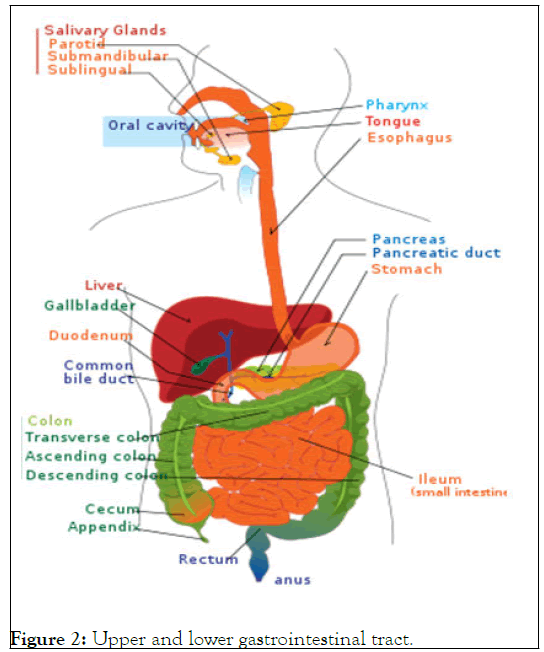
Figure 2: Upper and lower gastrointestinal tract.
Lower gastrointestinal tract: The majority of the small intestine and the entire large intestine are both parts of the lower gastrointestinal tract. Some sources claim that it also involves the anus.
Small intestine, which has three parts:
• Duodenum-Here the digestive juices from pancreas and liver mix together.
• Jejunum-It is the midsection of the intestine, connecting duodenum to ileum.
• Ileum-It has villi in where all soluble molecules are absorbed into the blood.
Large intestine, which has three parts:
• Cecum (the vermiform appendix is attached to the cecum).
• Colon (ascending colon, transverse colon, descending colon and sigmoid flexure)
• Rectum
• Anus
The upper and lower GI tracts are occasionally separated using the ligament of Treitz.
Myloelectric cycle
It is generally known that Sustained-Release (SR) dose forms can be administered to humans and animals via the stomach, acting as a sort of "depot."(2000) the stomach is anatomically separated into three sections: the fundus, body and antrum (pylorus).
While the antrum is the primary location for mixing motions and serves as a pump for stomach emptying by thrusting activities, the proximal portion made of the fundus and body serves as a reservoir for undigested material. Both when one is eaten and when one is fasting, the stomach empties. However, there are differences between the 2 states' motility patterns. An interdigestive series of electrical events occurs while fasting, cycling through the stomach and intestine every two to three hours. This is also known as the migrating myloelectric cycle or the inter digestive myloelectriccycle. When a mixed meal is consumed, the pattern of contractions switches from a fasted to a fed condition, which is also known as a change in the digestive motility pattern (2005) (Figure 3).
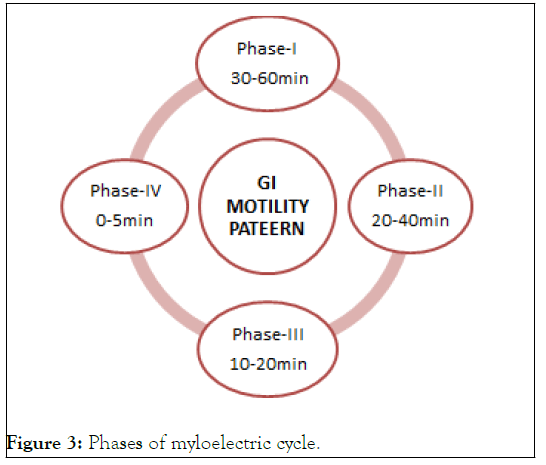
Figure 3: Phases of myloelectric cycle.
• Phase 1-(Basic phase)-30 to 60 minutes, with irregular contractions.
• Phase 2-(Preburst phase)-last 20 to 40 minutes while having sporadic contractions and action potential.
• Phase 3-(Burst phase) - consist of short, frequent and strong contractions that continue for 10 to 20 minutes.
• Phase 4-last happens between phase 2 and cycle 1 of two consecutive cycles, lasting 0 to 5 minutes.
In phase II of the fasting state, constant contractions are present in this pattern, which is also known as the digestive motility pattern. The size of the food particles is decreased by these contractions (to less than 1 mm) and they are then driven toward the pylorus in a suspension state. The fed state causes MMC to start later, which slows down the rate at which the stomach empties. Gastric emptying rates were determined using scanty graphic tests, which showed that controlled release dosage forms taken orally are primarily affected by two issues: short gastric residency duration and an unpredictably high gastric emptying rate (Table 1).
Approaches to design floating dosage form
Single-unit dosage forms: The globular shells in the low-density method can be employed as a drug carrier for a controlled release of the drug because they appear to have a lower density than gastric fluid. Another way to create a buoyant dose form is to use a fluid-filled system that floats in the stomach. Popcorn, poprice and polystyrol have all been used as drug delivery systems in coated shells. These shells have been undercoated with sugar polymeric materials such cellulose acetate phthalate and methacrylic polymer. A further layer of a drug-polymer mixture is applied to these. Depending on the kind of release required, ethyl cellulose or hydroxypropyl cellulose can be the preferred polymer. Finally, the product floats on stomach fluid while slowly delivering the medication over an extended period of time [6].
| Advantages | Disadvantages |
|---|---|
| Even at the alkaline ph. of the intestine, floating dose forms like tablets or capsules will stay in the fluid for extended periods of time | For medications that have issue with solubility or stability in stomach fluids, floating systems are not practical |
| Drugs intended for local action in the stomach such as antacids , benefits from FDDS | Drugs like Nifidipine, which undergo significant first pass metabolism & are well absorbed across the whole GI tract, may not be appropriate candidates for FDDS because prolonged stomach emptying may result in decreased systemic bioavailabity |
| To maintain the medicine in a floating state in the stomach & to obtain a substantially better reaction in cases of diarrhea and arduous gastrointestinal movement, FDDS dosage forms are favorable | One drawback of floating systems is that necessitate a high enough level of fluids in the stomach for the drug dosage forms to effectively float within |
| Aspirin & other similar medications should be administered with HBS/FDDS formulations because acidic substances like aspirin irritate the stomach wall when they come into touch with it | These systems also require the presence of food to delay their gastric emptying |
| Delivery of medications to stomach for local effect | Drugs that cause stomach mucosal irritation are not good candidates for FDDS |
Table 1: Advantages and disadvantages of FDDS.
A gas-filled floatation chamber may be incorporated into a micro porous component that contains a drug reservoir in fluidfilled floating chamber dosage forms. Along the top and bottom sides, there are openings or apertures through which gastrointestinal tract fluid can enter to dissolve the medicine. The undissolved medicine is kept inside by sealing the other two walls that come into touch with the fluid. Any suitable gas, liquid, or solid with the proper specific gravity and inert behavior can be used as the fluid in the presence, whether it be air, a partial vacuum, or another suitable fluid. The gadget is small enough to be swallowed, floats for a long time in the stomach and after complete release, the shell disintegrates, travels to the intestine, and is expelled. Hydro dynamically Balanced Systems (HBS) are created to increase absorption by extending the time the dose form spends in the gastro intestinal tract. Such systems are ideally suited for medications with superior acid solubility as well as medications with a particular site of absorption in the upper region of the small intestine. The dose form must have a bulk density of less than 1 in order to stay in the stomach for a long time. It need to remain in the stomach, keep its structural integrity and continuously release the medicine from the dose form. Chlordiazeopoxide hydrochloride serves as the best illustration of the effectiveness of the HBS capsule as a superior system. The medication is a classic example of a solubility issue because its solubility varies by a factor of 4000 from pH 3 to pH 6 (chlordiazepoxide hydrochloride is soluble in 150 mg/mL and 0.1 mg/mL at neutral pH).Three 10-mg commercial pills and HBS of chlordiazeopoxide hydrochloride exhibited equivalent blood level and time profiles. HBS can be produced as either a floating tablet or a capsule. By creating a drug delivery system with an asymmetric configuration, the 3-layer principle has been improved. This system allows for zero-order release kinetics and modulation of the release extent by initially maintaining a constant area at the diffusing front with subsequent dissolution/erosion toward the end of the release process. The device was made to float to extend stomach residence time in vivo, leading to a longer overall transit time inside the environment of the gastrointestinal tract with maximal absorptive capacity and therefore improved bioavailability. Drugs with pH-dependent solubility, a constrained window of absorption, and active transport absorption from either the proximal or distal region of the small intestine would fall under this particular description.
Single-unit formulations have a tendency to cling together or become clogged in the digestive tract, which increases the risk of causing discomfort.
Multiple-unit dosage forms: To create a dependable formulation with all the benefits of a single-unit form and none of the limitations of single-unit formulations listed above, multiple-unit dosage forms are designed. Many multiple-unit floatable dose forms have been created in an effort to achieve this goal. Numerous polymers, including albumin, gelatin, starch, poly methacrylate, polyacrylamine, and polyalkyl cyanoacrylate, have been employed with microspheres due to their high loading capacity. There have been created spherical polymeric micro sponges or "micro balloons." Microspheres exhibit great in vitro floatability and a distinctive interior hollow structure. Several devices with characteristics that extend, unfold, or are inflated by carbon dioxide generated in the devices after administration have been disclosed in the most current patent literature for carbon dioxide-generating multiple- unit oral formulations. If the diameter of these dose forms in their enlarged state is greater than between 12 mm and 18 mm, they are not allowed to pass through the pyloric sphincter (Figure 4) [7].
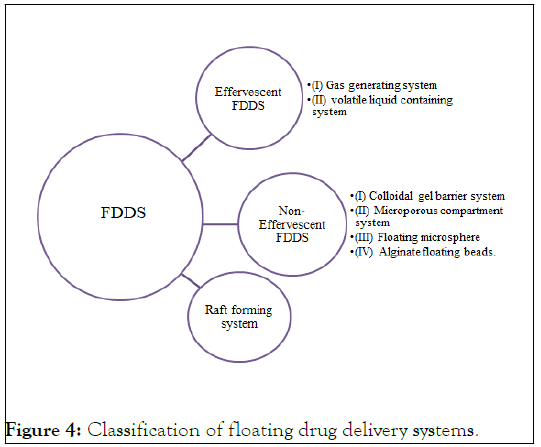
Figure 4: Classification of floating drug delivery systems.
Effervescent system FDDS: These systems are of the matrix kind prepared with the use of several effervescent chemicals and swellable polymers, including methylcellulose and chitosan. Ex: citric acid, tartaric acid, sodium bicarbonate. These are designed such that when they come into touch with gastric contents, CO2 is released and captured in swelling hydrocolloid, providing stability to the dose form.
Gas generating systems: They have a low density. The foundation of FDDS is the generation of CO2 inside the device after interaction with bodily fluids. The components are made so that when the dosage form enters the stomach, CO2 is liberated by the acidity of the gastric contents and is trapped in the gellified hydrocolloid, producing upward motion and maintaining the buoyancy of the dosage form. The dose form floats on the chyme due to a decrease in specific gravity. The CO2 generating components may be thoroughly combined inside the tablet matrix, in which case a single layer or bilayered is created with the medicine in the other layer prepared for a prolonged release effect and the gas generating mechanism in the first layer including hydrocolloid (Figure 5).
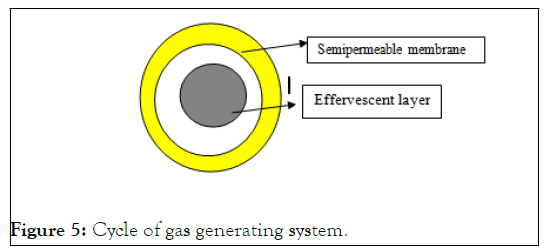
Figure 5: Cycle of gas generating system.
Volatile liquid containing systems: The apparatus, an osmotically regulated floating system, was made out of a hollow deformable unit that, after a considerable amount of time, could be raised from a collapsed posture. The housing, which was connected to the deformable unit, included a first chamber and a second chamber that were internally split by an impermeable, pressure-responsive movable bladder. The second chamber contains a volatile liquid, such as cyclopentane or ether, which vaporizes at physiological temperature to form a gas, allowing the drug reservoir to float. The first chamber contains an active drug. The gadget had a bio erodible plug that allowed the vapor to escape, allowing the unit to leave from the stomach.
Non-effervescent FDDS: Non-effervescent FDDS use matrixforming polymers such polycarbonate, polymethacrylate and polystyrene as well as hydrocolloids that can form gels or swell into cellulose. One type of formulation involves mixing the medication with hydrocolloids that form gel upon contact with gastric fluid following oral administration, maintaining shape integrity and a bulk density barrier. The air trapped by the swelling polymer gives the dosage forms buoyancy.
Colloidal gel barrier systems: Such a system contains medication with hydrocolloids that gel and are intended to float on the contents of the stomach. This extends GRT and maximizes the amount of drug that reaches the absorption site in solution form for ready absorption. This system incorporates a high level of one or more matrix-forming polymers like polycarbophil, polystyrene and polyacrylate as well as polysaccharides and gel-forming hydrocolloids of the cellulose type, such as (HPMC). The hydrocolloid in the system hydrates and forms a colloid gel barrier around its surface when it comes into touch with GI fluid.
Microporous compartment systems: A medication reservoir is enclosed inside a microporous compartment that has pores running the length of its top and bottom walls in order to implement this technique. To avoid any direct contact of the gastric surface with the undissolved drug, the periphery of the drug reservoir compartment is entirely sealed. The delivery system floats over the gastric contents in the stomach as a result of the flotation chamber's airtight seal trapping air. Through the opening, gastric fluid enters, dissolves the gastric fluid to the point where the medication cannot remain there and transports the drug in its dissolved state continuously across the intestine for absorption.
Floating microspheres/Micro balloons: The core hollow region within the microsphere makes hollow microspheres the most favorable buoyant system, according to experts. A unique emulsion solvent Diffusion approach was used to create hollow microspheres that are loaded with medication in their exterior polymer shelves.
Alginate beads/Floating beads: From freeze-dried calcium alginate, multi-unit floating dosage forms have been created. By adding sodium alginate solution to aqueous calcium chloride solution, spherical beads with a diameter of around 2.5 mm can be created. Causing calcium alginate to precipitate after being separated, the beads are quickly frozen in liquid nitrogen and then freeze-dried at 400°C for 24 hours, creating a porous structure that can sustain a floating force for more than 12 hours. The prolonged residence length of these floating beads was greater than 5.5 hours.
Raft forming systems: When a gel forming solution comes into touch with stomach fluid, it expands and produces a viscous, cohesive gel that traps trapped CO2 bubbles [8,9]. Raft forming systems have attracted a lot of attention for the administration of antacids and medicine delivery for gastro infections and disorders which creates a raft layer on top of the gastric fluid, releasing the medicine gradually into the stomach (Table 2).
| S.No. | Parameters | Conventional drug delivery system | Gastro retentive drug delivery system |
|---|---|---|---|
| 1 | Patient Compliance | poor | Better |
| 2 | Dose dumping | Risk of dose dumping is higher | No risk |
| 3 | Drug having low absorption in small intestine | Not appropriate | Appropriate |
| 4 | Drugs acting locally in the stomach | Not very much useful | Much useful |
| 5 | Toxicity | Greater susceptibility towards toxicity | Low susceptibility |
| 6 | Drugs with poor solubility at higher ph | Not Much beneficial | Much beneficial |
| 7 | Drugs that undergo degradation in colon | Not Much beneficial | Much beneficial |
| 8 | Drugs that have fast git absorption | Not Much beneficial | Much beneficial |
Table 2: Comparison between conventional and Grdds:
Mechanism of floating drug delivery systems
There have been several attempts to prolong the retention duration by keeping the dosage form in the stomach. These initiatives include the use of floating dosage forms, mucoadhesive dosage forms, high-density dosage forms, modified dosage forms, gastric-emptying delaying devices and the co-administration of gastric-emptying delaying medications [10]. The floating dose forms are the ones that are utilized the most frequently. As gastric fluids have a lower bulk density than Floating Drug Delivery Systems (FDDS), they float in the stomach without slowing down the rate at which the stomach empties. The medicine is slowly released from the system at the desired rate while floating on the gastric contents. The drug's residual system is expelled from the stomach after release. As a result, the oscillations in plasma drug concentration are better managed and GRT is raised. To keep the buoyancy of the dosage form on the surface of the meal, however, a minimal level of floating Force (F) is also necessary in addition to the minimal stomach content necessary to allow the effective attainment of the buoyancy retention effect. A unique apparatus for calculating the resultant weight has been described in the literature to measure the floating force kinetics. The device works by continually measuring the force F (as a function of time) that is needed to keep an object submerged (Figure 6) [11].
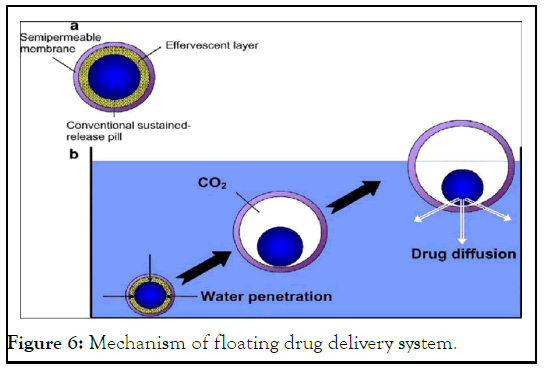
Figure 6: Mechanism of floating drug delivery system.
Factors influencing gastric retention
A number of factors can affect how long an oral dosage form stays in the stomach. The particle size should be between 1 mm and 2 mm in order to pass through the pyloric valve and into the small intestine. The pH of the stomach varies between 1.5 and 2.0 when someone is fasting and 2.0 to 6.0 when they are fed. The pH of stomach contents rises to 6.0 to 9.0 when a substantial amount of water is taken orally. Basic medications often have a better chance of dissolving in a fed condition than in a fasting one since the stomach doesn't have time to make enough acid when the liquid empties the stomach. Gastric emptying slows considerably in elderly individuals. Females empty their stomachs more slowly than males do. While depression slows it down, stress speeds up stomach emptying. The amount of liquid provided has an impact on how quickly the stomach empties. More liquid content means faster draining (Figure 7) [12].
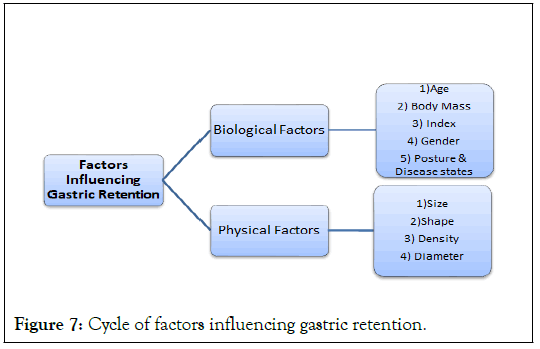
Figure 7: Cycle of factors influencing gastric retention.
When compared to all other shapes, ring-shaped devices had higher gastric rates. When compared to formulations with a 9.9 mm diameter, those with a diameter of more than 7.5 mm demonstrate a better stomach residence time [13]. The density of a dose form affects how quickly food leaves the stomach. A buoyant dose form whose density is lower than that of the contents of the stomach. As a result, the unit is kept in the stomach for an extended amount of time. When compared to single unit formulations, floating drug delivery system formulations have predictable gastric emptying patterns because the drug is freely distributed throughout the GIT (Tables 3-5).
| Microspheres | Granules | Films | Powders | Capsules | Tablets |
|---|---|---|---|---|---|
| Aspirin, griseofulvin | Diclofenac sodium | Cinnarizine | Several basic drugs | Chlordiazepoxide HCl | Acetaminophen |
| p-nitroaniline | Indomethacin | Drug delivery device | - | Diazepam | Acetylsalicylic acid |
| Ibuprofen | Prednisolone | - | - | Furosemide | Amoxycillin trihydrate |
| Terfenadine | - | - | - | L-Dopa and benserazide | Ampicillin |
| Tranilast | - | - | - | Misoprostol | Chlorpheniramine maleate |
| - | - | - | - | Propranolol HCl | Atenolol |
| - | - | - | - | Ursodeoxycholic acid | - |
Table 3: List of drugs explored for various floating dosage forms.
| Bioavailability hurdles | Therapeutics | Drug(s) |
|---|---|---|
| Local activity | Eradication of Helicobacter pylori | Amoxicillin |
| Narrow absorption window in upper GIT | Treatment of congestive heart failure, chronic renal failure and hepatic cirrhosis | Furosemide |
| Unstable in the colonic environment | Treatment of hypertension and congestive heart failure | Captopril |
| Narrow absorption window in upper GIT Short half-live | Treatment of hypertension, congestive heart failure, angina and arrhythmias | Metoprolol succinate |
| Short half-live local activity | Treatment of peptic ulcer and reflux esophagitis | Famotidine |
| Poor absorption from lower GIT | Treatment of hypertension | Atenolol |
| Low solubility at alkaline pH | Treatment of hypertension and tachycardia disturbances | Verapamil |
Table 4: Drugs suitable for gastro retentive drug delivery system.
| Tablets | Capsule | Microspher/Microparticles |
|---|---|---|
| Cellulosic hydrocolloids | Cellulosic hydrocolloids | Cellulose derivative |
| HPMC | HPMC | Ethyl cellulose |
| HPC | HPC | - |
| HEC | HEC | - |
| MC | NaCMC | - |
| NaCMC | - | - |
| Gel-forming hydrocolloids and matrix former | Gel-forming hydrocolloids and matrix former | Gel-forming hydrocolloids and matrix former |
| Carbopol | Sodium alginate | Eudragit |
| Carrageenan | Carbopol | Polycarbonate, |
| Gum guar | Agar | Polyacrylate, |
| Gum Arabic | Polymethacrylate | |
| Sodium alginate | - | Polystyrene |
| Polyethylene oxide | - | Chitosan |
| Polyvinyl lactam | - | Gelatin |
| Polyarcylates, | - | Alginate |
| Polyvinyl acetate | - | Gelucir |
Table 5: Polymers and other ingredients of floating drugs.
Evaluation of characterization parameters FDDS: For single unit dosage forms: (Ex: Tablets, Capsules).
Precompression parameters
Angle of repose: The angle of repose can be used to calculate the frictional forces present in grains or loose powder. This is the greatest angle that can be formed between a pile of powder or grains' surface and the horizontal plane. Using a cathetometer, the height (h) of the resulting heap is measured, together with the radius (r) of the cone's base. Calculating the angle of repose () requires (Table 6 and Figure 8).
θ= tan-1 [h/r]
Where θ is angle of repose
| Angle of repose | Powder flow characterstics |
|---|---|
| <25 | Excellent |
| 25-30 | Good |
| 30-40 | Passable |
| >40 | Very poor |
Table 6: Table of angle of repose.
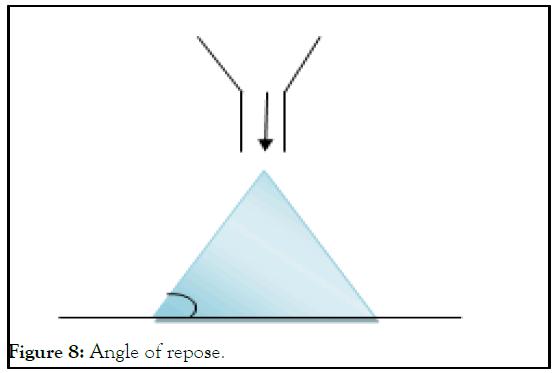
Figure 8: Angle of repose.
Carr’s compressibility index: A precise weight of the formula blend is poured into a volumetric cylinder to fill the volume (V0) and it is then tapped repeatedly into a solid surface until the volume remains constant (Vf) "% compressibility" of Carr.
Compressibility Index (CI)=V0-Vf/V0 × 100
Density: The density is an important parameter for floating pellets. The pellet would floats only when its density is less than that of gastric fluid (1.004). The density is determined using following relationship.
V=r2hd=m/v
v=Volume of tablet (cc)
r=Radius of tablet (cm)
h=Crown thickness of tablet (g/cc)
m=Mass of tablet
Post compression parameters
Hardness: A tablet's hardness reveals its capacity to tolerate managing mechanical shocks. Using the Monsanto hardness tester, the tablets' hardness can be assessed. It is stated as kg/ cm2.
Friability: The friability of tablets was determined by using Roche Friabilator. It was expressed in percentage (%). Ten tablets were initially weighed (W initial) and transferred into friabilator. The friabilator was operated at 25 rpm for 4 minutes or run up to 100 revolutions. The tablets were weighed. % Friability of tablets less than 1% was considered acceptable.
F(%)=(Wi-Wf/Wi) × 100
Weight variation: The weight variation test is conducted in accordance with the official monograph's instructions. The U.S.P. limitations have been calculated (Table 7).
| Average weight of tablet, pellets | Percent deviation |
|---|---|
| 130 mg or less | 10 |
| >130 mg but <324 mg | 7.5 |
| 324 mg or more | 5 |
Table 7: Table on weight variation.
Determination of the drug content: The amount of medication included in the pellet formulations was determined using 0.1 N HCl for extraction. A volumetric flask was filled with modified release pellets of the material (100 mg), which were effervescent layered pellets coated with a polymeric membrane that trapped gas. To achieve a thorough extraction, 0.1 N HCl was added to the mixture and the mixture was then subjected to sonication for 30 min [14]. After being passed through filter paper, being properly diluted with 0.1 N HCl and being measured spectrophotometrically at nm, Between 2 g/ml and 20 g/ml, Beer's Law could be seen throughout the analysis. There was a calculation of the drug content.
Floating studies (In vitro buoyancy studies: The in-vitro floating research was conducted by adding 10 mL of formulation to 100 mL of 0.1 N HCl, (pH 1.2), at 37°C. Without much disruption, the formulation's time to first appear on the dissolution media surface (floating lag time) and its duration of floating the amount of time it stayed there continuously were both recorded. The following equation was used to calculate the percentage of floating [15].
Floating pellets (%)=Number of floating pellets at the measure time/Initial number of the pellets × 100
Dissolution study (In vitro drug release study): The USP apparatus II (paddle method -37.0 ± 0.5°C, 50 rpm, 500 ml, 0.1 N HCl, n=3) was used to study the in vitro release of sample pellets (simple drug) and with drug loaded pellet formulations. At predetermined intervals, 1 ml of sample was removed, and the same volume of fresh medium (37°C ± 0.5°C) was added right away. The absorbance of the samples was measured at nm using a UV spectrophotometer to determine the quantity of sample pellets released. The total number of sample pellets released was then computed [16,17].
Scanning Electron Microscopy (SEM): A scanning electron microscope was used to look at the pellets' surface morphology with the help of double-sided adhesive tape; the pellets were attached to the stubs. Under an argon environment, gold and palladium were sputtered onto the mounted samples, which were then subjected to accelerating voltage measurements at 20 kV (Table 8).
| Brand name | Drug |
|---|---|
| Madpar (HBS) | Benserazide |
| Almagate | Chlorazepam |
| Valrelease | Diazepam |
| Valium pills | Diazepam |
| Topalkan | Alginic acid |
Table 8: Table on electron microscopy.
The review briefly describes the mechanism, types of floating system, advantages, limitation, factors affecting floating system, drug candidates suitable for floating, evaluation parameters and application of the system. These systems are useful to several problems encountered during the development of a pharmaceutical dosage form and the future potential of FDDS. The advantages and future prospects for oral controlled drug delivery of the present technological breakthroughs of FDDS, including patented delivery methods and marketed products, are described in this review.
[Crossref] [Google Scholar]] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Schoalr] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Schoalr] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Mali KD, Baviskar AR, Patil SS, Saner HS (2023) A Comprehensive Review on Floating Drug Delivery System. J Appl Pharm Sci. 15:361.
Received: 14-Mar-2023, Manuscript No. JCRIO-23-22137; Editor assigned: 17-Mar-2023, Pre QC No. JCRIO-23-22137(PQ); Reviewed: 31-Mar-2023, QC No. JCRIO-23-22137; Revised: 09-May-2023, Manuscript No. JCRIO-23-22137(R); Published: 06-Jun-2023 , DOI: 10.35248/1920-4159.23.15.361
Copyright: © 2023 Mali KD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.