
Advances in Pediatric Research
Open Access
ISSN: 2385-4529

ISSN: 2385-4529
Research Article - (2024)Volume 11, Issue 1
Objective: Sepsis is one of the most important and serious infection with high mortality in childhood. The aim of this study was to determine the serum level of vitamin A and zinc in children with sepsis compared to control group.
Methods: This case-control study was performed on 40 septic children and 25 controls admitted to Rasoul-e-Akram and Ali Asghar hospitals affiliated by Iran University of Medical Sciences in Tehran from July 2015 to September 2016. The case group included children less than 5 years old with septicemia and the control group consisted 25 children without infection (healthy children undergone for elective surgery). 5 ml of blood was taken from both groups. The amount of vitamin A was determined by High-Performance Liquid Chromatography (HPLC) method and zinc levels determined by calorimetric method. The student’s t test, chi square and other tests by Statistical Package for the Social Sciences (SPSS) software, version 13.5 were used. Probability (P) values lower than 0.05 were considered significant in this trial.
Results: Seven of 40 patients died due to severity of disease and septic shock. 33 cases (mean age of 58.4 years), 60.6% male, 39.4% female and control group were evaluated. Sex (P=0.8) and mean age (P=1.000) were not significantly different between two groups. 57.6% of patients with sepsis had zinc deficiency and 42.4% had vitamin A deficiency, but none controls had deficiencies. Vitamin A cut off level 0.25, had 75% sensitivity and 48% specifity. Zinc serum cutoff level 0.605 had 75% sensitivity and 40% specifity to differentiate the septic cases from control group.
Conclusion: Based on the current study, a significant percentage of young septic children (<5 years old) were deficient for zinc (57.6%) and vitamin A (42.4%) but none of controls. Although low levels of vitamin A in our children (based on previous studies) do not play a significant role in the development of limited bacterial infections (respiratory, gastrointestinal, urinary), it is observed that the deficiency of both zinc and vitamin A in young studied children predispose them to a severe form of bacterial infections and increases the risk of sepsis and mortality rate. So adding vitamin A and zinc to children's diets will reduce sepsis risk.
Zinc; Fever; Diarrhea; Children; Febrile seizures
Sepsis is one of the most critical and severe childhood infections and one of the significant causes of death in young patients. Delay in treatment will result in high mortality in septic cases. Therefore, empirical treatment should be started as soon as possible based on the most common responsible organisms. The incidence of sepsis and septic shock has increased over the last 20 years due to several causes for immune deficiency states (e.g. increased life expectancy, underlying diseases, Acquired Immunodeficiency Syndrome (AIDS), corticosteroids, various catheters and mechanical and artificial respiration devices).
Increased use of antimicrobials has also increased sepsis prevalence [1-3]. Despite of broad clinical spectrum (decreased tissue perfusion) and metabolic symptoms (in end organs: heart, lung, brain, liver, kidney) are considered for diagnosis of sepsis. Signs of fever and Systemic Inflammatory Symptoms (SIRS) such as tachypnea, tachycardia, leukocytosis or leukopenia and fever or hypothermia are valuable in young septic cases. If SIRS progresses to multiple organs involvement (severe sepsis), septic shock and death is the final stages. Sepsis symptoms are nonspecific in children. Bachuret, et al., [3] study reported a predictive model for serious bacterial infections among infants younger than 3 months of age. Craig, et al., [4] in a prospective cohort study demonstrated the accuracy of clinical symptoms and signs for the diagnosis of serious bacterial infection in young febrile children. The increased hospital admission rates for febrile neonates reported as high as 20% but recent studies cite lower rates with 2.8% to 3.1% for bacteremia and 1% or lower for bacterial meningitis. WU, et al., [5] had done a retrospective cohort study in febrile neonates (1 to 28 days old) in North Carolina. Hospitalizations for febrile neonates from the emergency department ranged from 78% to 84%. Probably the variation is due to testing, treatment and disposition from various studies. Chime, et al., [6] addressed differences in the management of fever and febrile seizures between and pediatrics department. Hospitalizations for febrile infants and neonates from the emergency department ranged from 78% to 84%. Despite the guidelines that advise admission, 54% of pediatric emergency directors report full compliance with these published guidelines. Most of previous original guidelines studies such as the Boston, Philadelphia and Rochester studies are >20 years old. Various algorithms and guidelines have been developed for febrile infants. Bacterial sepsis is one of the most common causes of sepsis in children and positive blood culture can be a sign of diffuse blood infection and sepsis. There is no laboratory or clinical test for definitive diagnosis of sepsis in all cases. Sometimes, sepsis can be resulted from bacterial toxin or antigens in cases with negative blood culture. Van den, et al., [7] in a systematic review defined the diagnostic value of laboratory tests in identifying serious infections in febrile children. Gomez, et al., [8] in a prospective multicenter study demonstrated the diagnostic value of leukopenia in infants (<9 days) with fever without source. Isolation of S aureus- DNA and detection of Toxic Shock Syndrome (TSST) reported by some authors [9,10]. Recently, American Academy of Pediatrics is currently conducting a nationwide quality improvement project to reduce variability in infant sepsis evaluation that reinforces that neonates be admitted regardless of risk factors. Different factors influence the severity of infection such as causative pathogen and the host immune response [1-3]. Zinc and vitamin A supplements are already recognized as simple and affordable methods to reduce incidence and severity of diarrhea [11-15]. Castellani, et al., [11] defined the relationship between vitamins and cytokines in immunity. Vitamins and micronutrients especially zinc play an important part in supporting immune system and their homeostasis has a crucial importance in response to infections. Effects of vitamin A deficiency on mucosal immunity was explained by Gogia [12]. Cameron, et al., [11] determined the vitamin A deficiency and its impact on acute respiratory infections among preschool Inuit children. According to Kartasurya, et al., [14] in a randomized trial study in preschool children in Indonesia, zinc combined with vitamin A reduces upper respiratory tract infection morbidity. Gunay, et al., [15] reported the serum zinc, copper levels and copper/zinc ratios in infants with sepsis syndrome. Bhandari, et al., [16] studied the effect of routine zinc supplementation on pneumonia in children in a randomized controlled. According to Dibley, et al., [17] study, vitamin A supplementation fails to reduce incidence of respiratory infection illness and diarrhea in preschool-age Indonesian children. Akhtar, et al., [18] reported the micronutrient deficiencies in South Asia. Vitamin A and other micronutrient deficiencies had a significant cause of malnutrition in preschool children in developing countries especially in South Asia.
Sepsis and other bacterial infections (e.g. Urinary Tract Infection (UTI), Acute Gastroenteritis (AGE), Upper Respiratory Infection (URI)) are considered as the most common causes of hospital admission in Iranian young children [19,20].
Like as other Middle East and Asia countries, nutritional deprivation (poor and inadequate diet) both for micronutrients and vitamins (especially vitamins A and D, zinc, iron) are common in Iranian children. Finding from a recent national study in Iran demonstrated vitamin D, retinol and zinc deficiencies with stunting in toddlers [21-28].
To assess the relation, if any between low serum concentration of vitamin A and zinc in developing sepsis, we carried out this prospective case-control study. This study aimed to determine the serum levels of vitamin A and zinc in children with sepsis compared to the controls.
This case-control study from July 2015 to September 2016 in patients admitted to the pediatric ward of Hazrat Rasoul Akram and Ali Asghar hospitals in Tehran. After approval by the ethics committee of the Pediatric Infectious Diseases Research Center affiliated to Iran University of Medical Sciences, code 9311165017. Completing the agreement form was done by the parents. The experiments were performed at no additional cost. The project stages were ethical in medical research and commitment to the principles of the Helsinki Convention.
Case definition
The patient samples included 40 admitted children (less than five years old) hospitalized in the pediatric wards diagnosed as sepsis based on their history, clinical examination and course of the disease.
Exclusion criteria
We excluded all cases with severe malnutrition, immunodeficient cases known malignancies or malabsorption states. All febrile children diagnosed with other non-infectious causes (after clinical examinations and additional tests) were excluded from the study.
Controls
The control group consisted of 25 children (<5 years old) who were hospitalized in the surgical ward for elective surgery. They were visited by a pediatrician before surgery to be assessed free of infectious status. Only if they had no manifestation of the disease after appropriate physical exams, they were considered as controls. We used their extra blood (which was taken for their routine blood tests before their respective surgery) for lab tests.
Lab tests
Blood culture and bacterial determination were performed in septic cases. 5 ml of blood was collected from 2 groups for routine tests and study. Serum was separated and stored in the freezer under appropriate conditions until the experiments were performed. Vitamin A and zinc levels were determined in the sample. Serum zinc level was measured by spectrophotometry (GBC scientific, USA). Zinc level <63.8 mic/dl were considered zinc deficient. High-Performance Liquid Chromatography (HPLC) was used to determine vitamin A level (retinol). Vitamin A deficiency was determined based on age (normal range for age 1-6 years old was 0.2-0.4) amount below 0.2 mic/ml were considered vitamin A deficient. Serum zinc and vitamin A levels in children with sepsis (case group) were evaluated and compared with the control group.
Static analysis
All the data obtained from the questionnaires were analyzed by Statistical Package for the Social Sciences (SPSS) software version 20. Descriptive statistical methods were used to determine the frequency and analytical, statistical methods including the χ² test and student's t-test, were used to compare serum levels of vitamin A and zinc between groups. P<0.05 was considered significant. Receiver Operating Characteristic (ROC) curve illustrated for determination of sensitivity, specifity and positive, negative predictive values.
During this study, 153 patients with initial examinations upon admission to the ward were suspected of having sepsis. After completing their tests and subsequent examinations, 89 people were diagnosed with other diseases and finally eliminated. Of the remaining patients with suspected sepsis, only 47 met the inclusion criteria. Seven of them died and no further tests were possible. In 7 patients, there was not enough blood sample to test for vitamins and zinc. Finally, 33 patients were thoroughly evaluated (Figure 1).
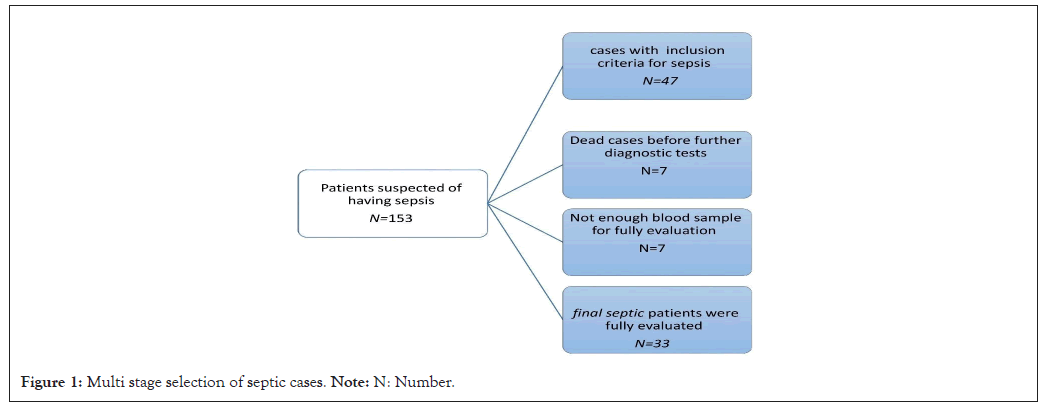
Figure 1: Multi stage selection of septic cases. Note: N: Number.
Twenty-five children in the control group were also examined. Age and gender distribution were compared between 2 groups. A total of 20 patients (60.6%) were male and 13 patients (39.4%) were female with a mean age of 4.58 ± 5 years. There was no apparent difference between patient and control in terms of sex (P=1) and age (6.7 ± 2.9 in the control group and 4.8 ± 5 years in the patient group (P=0.08) (Tables 1 and 2).
| Group | Gender | |
|---|---|---|
| Male | Female | |
| Patients | 60%/6 (n=20) | 39%/4 (n=13) |
| Control | 60% (n=15) | 40% (n=10) |
| P-value | 1 | |
Note: P: Probability. Chi-square <0.05 was considered significant.
Table 1: Comparison the gender between cases and controls.
| Group | Number of patients | Mean | Standard deviation | P-value | |
|---|---|---|---|---|---|
| Age | Case | 33 | 4.8485 | 5.01947 | 0.056 |
| Control | 25 | 6.72 | 2.90861 | ||
Note: P: Probability.
Table 2: Comparison the age between cases and controls.
All the clinical and laboratory variables (mean age, length of hospital stay, White Blood Cells (WBC), Erythrocyte Sedimentation Rate (ESR)) are demonstrated in Table 3.
| Variables | Age (years) | Days of hospitalization | ESR (Mm/hr) | WBC (1000/ml) | Zinc ( µg/dl) | Vitamin A ( µg/dl) |
|---|---|---|---|---|---|---|
| Mean | 4.84 | 6.24 | 39.9 | 12.08 | 77 | 0.28 |
| Standard deviation | 5.01 | 2.72 | 3.02 | 5.41 | 4.16 | 0.12 |
Note: ESR: Erythrocyte Sedimentation Rate; WBC: White Blood Cells.
Table 3: Clinical and laboratory variables in septic children.
Distribution of zinc and vitamin A level in controls and septic cases is shown in Figures 2-5.
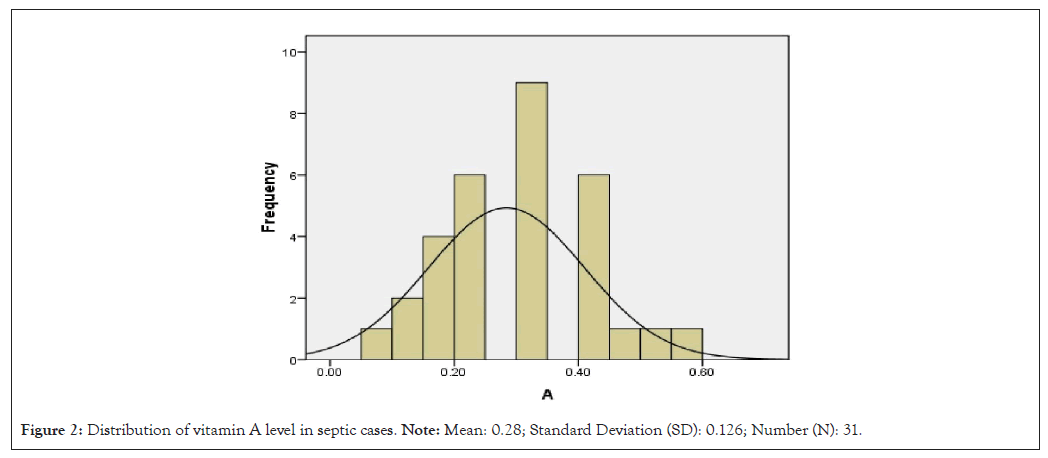
Figure 2: Distribution of vitamin A level in septic cases. Note: Mean: 0.28; Standard Deviation (SD): 0.126; Number (N): 31.
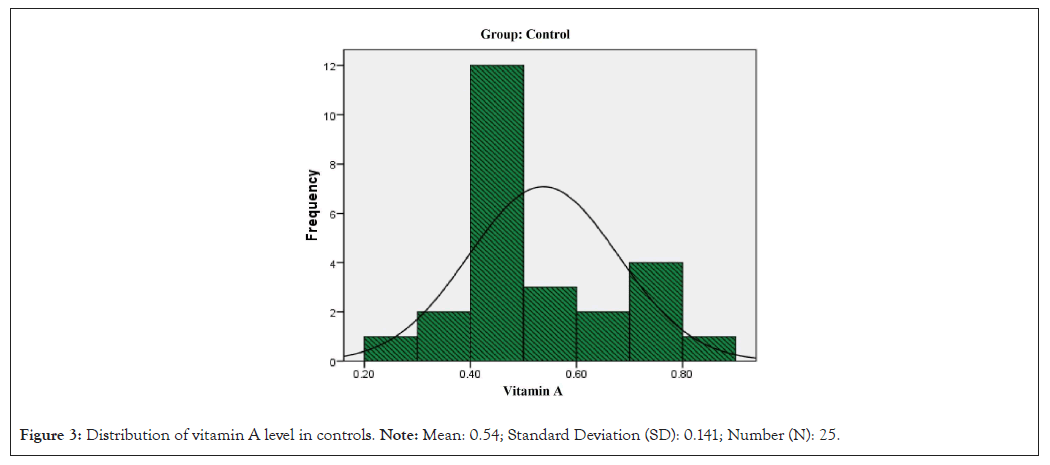
Figure 3: Distribution of vitamin A level in controls. Note: Mean: 0.54; Standard Deviation (SD): 0.141; Number (N): 25.
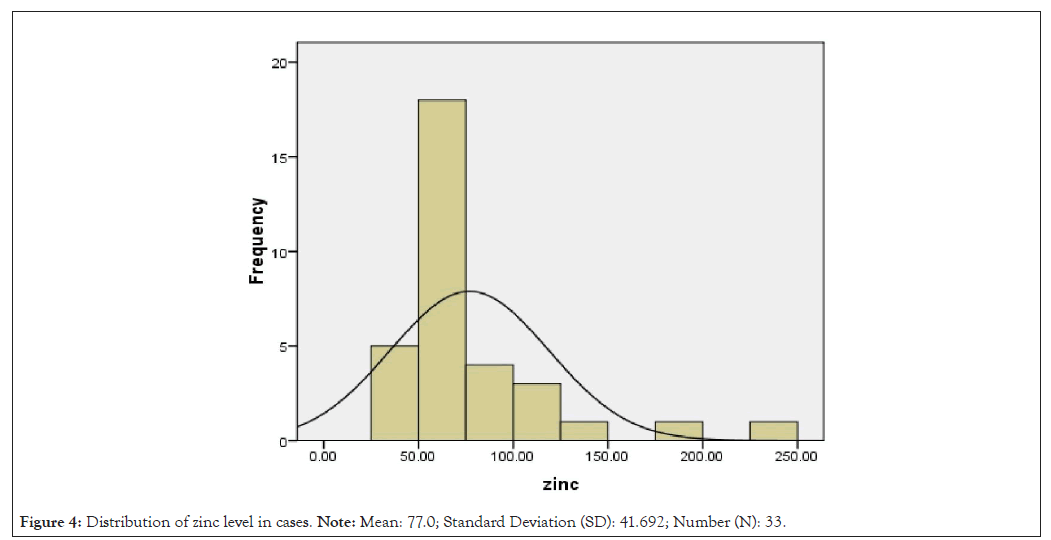
Figure 4: Distribution of zinc level in cases. Note: Mean: 77.0; Standard Deviation (SD): 41.692; Number (N): 33.
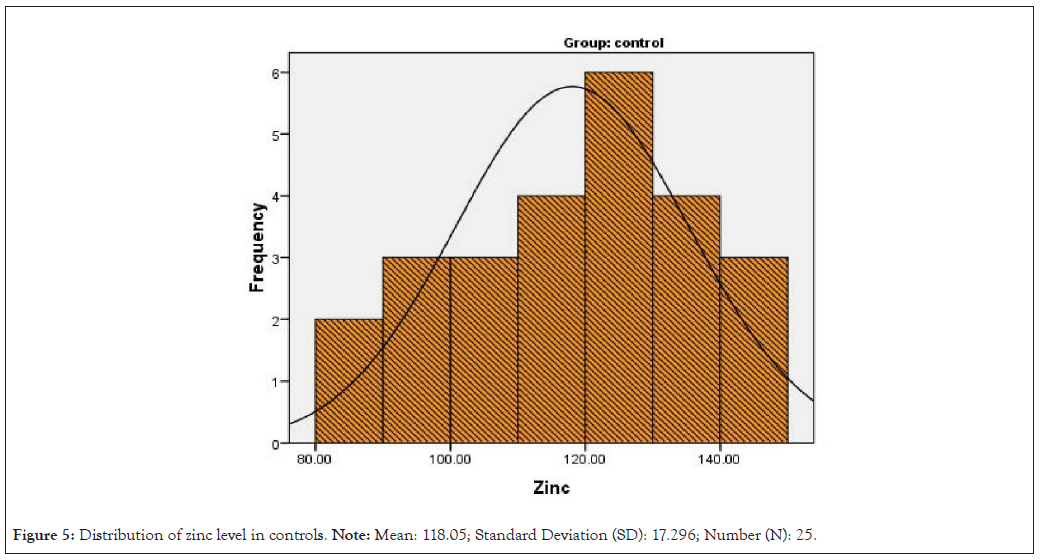
Figure 5: Distribution of zinc level in controls. Note: Mean: 118.05; Standard Deviation (SD): 17.296; Number (N): 25.
Comparison of vitamin A and zinc levels in children with sepsis and controls showed in Table 4. In septic children, the mean zinc level was 77 ± 4.16 and vitamin A was 0.280 ± 0.12 mg/ dL. 57.6% (19/33) of septic cases were zinc deficient and 42.4% (15/31) were vitamin A deficient (compared to typical values based on patient age).
| Variables | ||||
|---|---|---|---|---|
| Vitamin A ( µg/dl) | Zinc ( µg/dl) | |||
| Groups | Patients | Controls | Patients | Control |
| Mean | 0.28 | 0.54 | 77 | 118.05 |
| Standard deviation | 0.12 | 0.14 | 41.6 | 17.29 |
| P-value | 0.4 | 0.05 | ||
Note: P: Probability. Chi-square <0.05 was considered significant.
Table 4: Comparison of vitamin A and zinc levels in children with sepsis and controls.
Based on the ROC curve, Area Under the ROC Curve (AUC) for zinc was 0.601 (0.372-0.829, P=0.05). Zinc serum with a cut-off of 0.605 had 75% sensitivity and a 40% specificity in differentiating the septic cases from controls. The AUC for vitamin A: 0.562 (0.313-0.812, P=0.4). Vitamin A with a cut-off of 0.25 had 75% sensitivity and a 48% specifity to differentiate between 2 groups (Figure 6 and Tables 5 and 6).
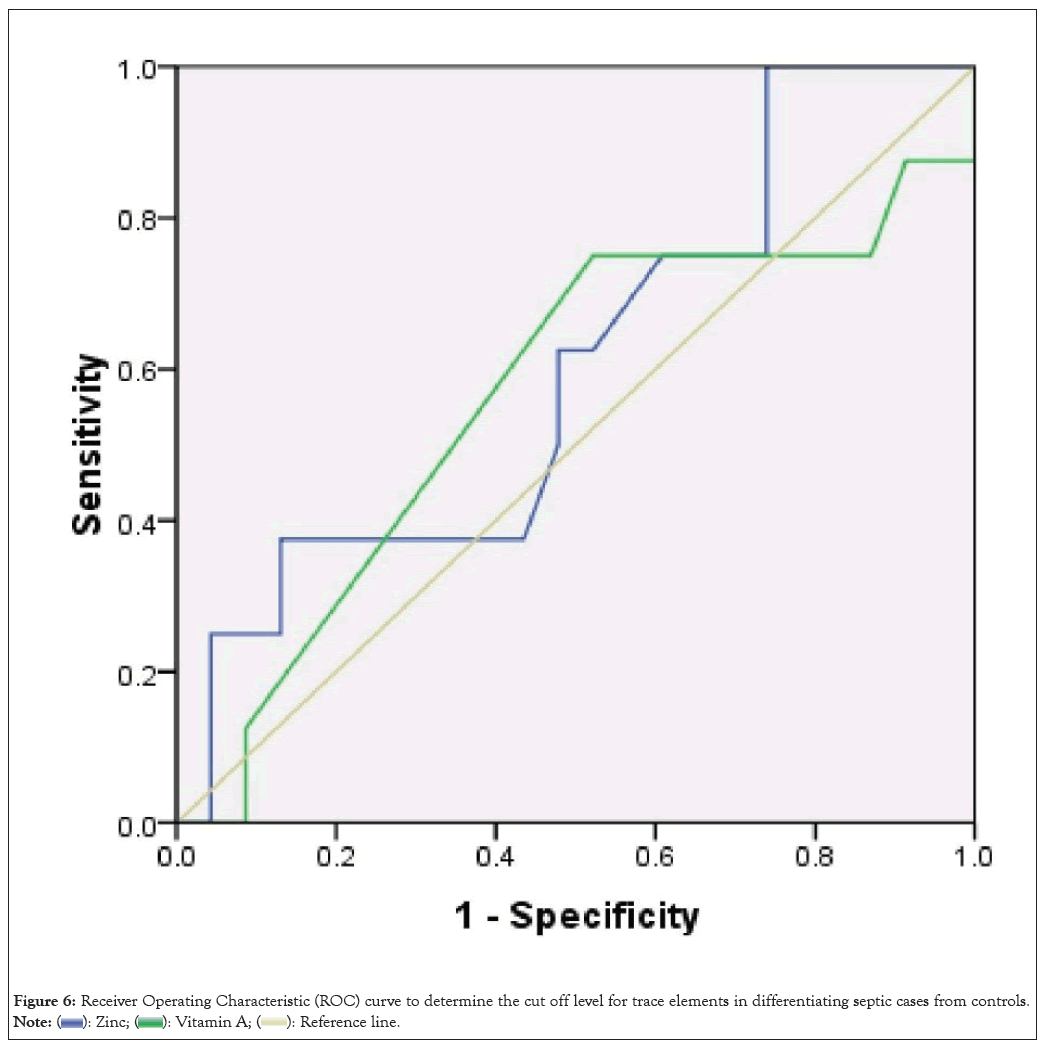
Figure 6: Receiver Operating Characteristic (ROC) curve to determine the cut off level for trace elements in differentiating septic cases from controls. 
| Test result variable(s) | Area | Standard Errora (SE) | Asymptotic significanceb | Asymptotic 95% Confidence Interval (CI) | |
|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||
| Zinc | 0.601 | 0.116 | 0.404 | 0.372 | 0.829 |
| Vitamin A | 0.562 | 0.127 | 0.604 | 0.313 | 0.812 |
Note: (a): Under the non-parametric assumption; (b): Null hypothesis (true area: 0.5).
Table 5: Interpretation of Area under the curve.
| Test result variable(s) | Positive if ≥ a | Sensitivity | 1-Specificity |
|---|---|---|---|
| Zinc | 28 | 1 | 1 |
| 32 | 1 | 0.957 | |
| 40 | 1 | 0.913 | |
| 46.5 | 1 | 0.87 | |
| 49 | 1 | 0.826 | |
| 51.5 | 1 | 0.783 | |
| 53.5 | 1 | 0.739 | |
| 56 | 0.75 | 0.739 | |
| 60.5 | 0.75 | 0.609 | |
| 63.5 | 0.625 | 0.522 | |
| 64.5 | 0.625 | 0.478 | |
| 65.5 | 0.5 | 0.478 | |
| 66.5 | 0.375 | 0.435 | |
| 67.5 | 0.375 | 0.391 | |
| Vitamin A | 0 | 1 | 1 |
| 0.09 | 0.875 | 1 | |
| 0.13 | 0.875 | 0.913 | |
| 0.165 | 0.75 | 0.87 | |
| 0.185 | 0.75 | 0.783 | |
| 0.25 | 0.75 | 0.522 | |
| 0.35 | 0.375 | 0.261 | |
| 0.435 | 0.125 | 0.087 | |
| 0.485 | 0 | 0.087 | |
| 0.55 | 0 | 0.043 | |
| 1 | 0 | 0 |
Note: (a): Under the non-parametric assumption.
Table 6: Interpretation of the coordinates of the curve.
The present study results showed that approximately 60% of septic children diagnosed as zinc deficient and 40% were vitamin-A deficient but none of the healthy children (control) were deficient. Based on the ROC curve, AUC for zinc was 0.601 (0.372-0.829, P=0.05). The AUC for vitamin A is 0.562 (0.313-0.812, P=0.4). However, vitamin A level=0.25 had 75% sensitivity and 48% specificity. Also, the serum zinc level=0.605 had 75% sensitivity and 40% specificity to differentiate control children from septic ones. However, vitamin A and zinc deficiency in children (<5 years old) exposed the children to severe bacterial infections and sepsis.
Different factors influence the severity of infection such as causative pathogen and the host immune response. About 2 million children die annually as a result of respiratory tract infection. Even in developed countries, the prevalence of these infections is very high. Around 19 to 27% of annual hospitalizations for children younger than 15 is caused by acute respiratory tract infection. In 2009, World Health Organization (WHO) report, diarrhea was the second leading cause of death in children younger than 5 years. Almost one in every 5 deaths in children (1.5 million annually) is due to diarrhea. A meta-analysis of 25 studies found that giving children vitamin A reduced the risk of measles. By adding zinc to the diet of children under 5 years of age, it was observed that the incidence of pneumonia in children decreased. The effects of postnatal vitamin A administration in Guinea-Bissau were examined [2- 5]. They monitored 4,183 children after 28 days to 6 months of age and found that in 165 cases of measles, children receiving vitamin A were less likely to be hospitalized and less likely to die than boys (not girls). A meta-analysis of 30 studies showed that adding vitamin A to adults' diet did not affect Human Immunodeficiency Virus (HIV) progression but reduced mortality in African children and reduced deaths from diarrhea and lung infection in children [6]. Studies showed that vitamin A deficiency and increasing respiratory infection exacerbate wheezing in infants [7]. The severity of the visa and its duration depend on the severity of vitamin A deficiency. Children under 5 years of age were found to have vitamin A deficiency as a significant risk factor for acute otitis media and lower respiratory infections [8]. In contrast, other studies were in the opposite direction, including Gogia [12] reviewed 7 published studies and concluded that vitamin A's addition to the infant's diet. However, not side effects did not reduce respiratory infections [9]. Many interventional trails had investigated vitamin A positive influence on respiratory tract infections [10]. According to their results high doses of vitamin A reduces the number and severity of acute non measles respiratory infections. Besides rickets, other complications have recently been reported as results of vitamin D deficiency [6,7]. The relationship between vitamin D and acute respiratory tract infections was shown in a cross-matched prospective trial and other case-control studies. Masironi [13] reported the epidemiologic distribution of trace element deficiencies in world [10-12]. Clinical vitamin D deficiency and Rickets increases the risk of pneumonia in Ethiopian children younger than 5 years by 13 times. Another study in Yemen showed that 50% of children hospitalized due to acute respiratory infections had also vitamin D deficiency.
Kartasurya, et al., [14] investigated the effect of adding zinc to the diet before and after vitamin A supplementation in children aged 2-5 years in the endothelium. He found that adding zinc and vitamin A to preschool children reduced the number of days of respiratory infections. Bhatnagar and colleagues [12] examined children under 4 months of age with diarrhea and found that children receiving zinc had lower therapeutic failure rates than the control group. By adding zinc to the diet of children under 5 years of age, studies found that diarrhea in children decreased. In a meta-analysis, it was noted that zinc consumption reduced diarrhea incidence by 9% and was even able to prevent recurrent recurrences of diarrhea by 28%, he concluded that there were many inconsistencies in the findings of various studies. It can be caused by differences in the age of the people under study or the study's content and even the type of zinc used. Studies showed that it is recommended to add vitamin A to children with low nutritional levels and weight [14]. In a study in India, studies reported pneumonia as the cause of 1.4 deaths in children under 5 in India [15]. They noted a higher risk of death in younger children and malnutrition. In their study, little evidence has been provided to support the prophylactic or therapeutic effect of vitamin A in pneumonia. 7 experimental studies in children under 5 years of age with acute lower respiratory infection in developing countries were performed [16]. There was no difference in wound healing speed and the length of hospital stay in the zinc-receiving and control groups. Bacterial infections (sepsis, UTI and AGE) is considered as one of the most common causes of hospital admission in Iranian young children [12-18]. Akhtar, et al., [13] reported the micronutrient deficiencies in South Asia [17]. Like as other Asian countries nutritional status in Iranain population might has some roles in recurrent infections in Iranian children [17,21-26]. The role played by micronutrients in upper and lower respiratory infections was shown in Iranian children before. Many studies have been carried out on the role of several trace elements either individually or in combination/antagonism in the pathogenesis and etiology of various diseases. Results of a recent national study in Iran (2020) upon association of vitamin D, vitamin A and zinc deficiencies with stunting in toddlers (mean age=19.2 ± 8.4 months) published in recent year [25]. A significant inverse association was found between serum vitamin A concentrations and the odds of stunting. Also, a significant inverse association was found between serum levels of vitamin A and stunting in girls and those who did not use nutritional supplements. Although vitamin D levels were not significantly associated with stunting in the overall study population. But, they found a positive association among toddlers who used nutritional supplements. No significant association was found between serum levels of zinc and stunting [25]. Effect of zinc deficiency on the risk of urinary tract infection in Mashhad, Iran in 2020 was described by Zabihi, et al., [26]. According to above study, serum zinc level in UTI children were lower than controls (60.0 ± 17.1 μg/dl vs. 83.0 ± 15.7 μg/dl; P=0.001). After being adjusted for demographic factors, the zinc deficiency proved to be a significant predictor of UTI (Odds ratio (OR)= 8.633, 95% confidence interval=3.084-24.171, p<0.001). It concluded that zinc deficiency can put the children at an eight times higher risk for developing UTI, independent of age and gender [26]. The role of zinc deficiency in febrile seizures in Isfahan was also investigated [23]. According to Mohsenpour, et al., [22] upon female cases with recurrent urinary tract infections, the zinc level in cases was significantly lower than controls (76.72 ± 17.06 vs. 96.83 ± 11.25 p=0.01). Level of zinc reduced with aging. It seems that zinc levels are a risk factor for recurrent urinary tract infections [21]. Yousefi, et al., [21] evaluated he therapeutic role of zinc in urinary tract infections. They concluded that zinc supplementation has a significant effect in ameliorating severe dysuria and frequency of urinary in UTI. When the abdominal pain is present, usage of this drug is not recommended.
In recent years at least 4 sequential prospective studies had done in hospitalized children in pediatric ward of Rasul Akram hospital, Tehran, Iran [21-24].
The mean and standard deviation of serum selenium concentration in children with common infections (75 Respiratory Tract Infection (RTI): 25, AGE: 25 and UTI: 25) was compared with 40 normal control cases aged between 6 months-5 years (mean: 2.17 years). The mean and standard deviation of serum selenium concentration of Gastrointestinal Infection (GI), RTI, UTI and control groups were 64.70 ± 21.43 mg/l, 61.60 ± 19.25 mg/l, 66.37 ± 22.11 mg/l and 62.20 ± 22.08 mg/l, respectively without significant differences in serum selenium levels between these groups (P value=0.608).
According to one trial upon UTI, a good correlation between vitamin A level and progression of UTI in children had found in our country. The effect of vitamin A prevention the renal damage following acute pyelonephritis in Iranian children reported Ayazi, et al., [29]. The median of serum ferritin levels in GI, RTI, UTI and control groups were 60.05 (48.82-78.01), 62.00 (49.07-79.35), 60.60 (51.78-79.52) and 58.75 (45.32- 76.72) respectively.
The difference in ferritin levels between these groups was statistically significant (P-value<0.001). Compared with the control, the RTI and GI groups had significantly higher levels (P<0.001). However, the UTI group was not statistically different from the control (P=0.098). None of the children had ferritin constrictions below 12 [21,30].
In contrast to the present study, no clear zinc deficiency was observed in AGE patients. The mean zinc level in AGE was 102 vs. 118 in the control group; P-value=0.1. Indeed, vitamin A deficiency was not observed in any of the patients with AGE. Like here, zinc deficiency has been implicated in the development of pneumonia in hospitalized children, such as sepsis. But vitamin A deficiency did not play a role in causing pneumonia [19-22].
The role of zinc deficiency in febrile seizures in Isfahan was also investigated [23]. According to Mohsenpour, et al., [22] upon female cases with recurrent urinary tract infections. The zinc level in cases was significantly lower than controls (76.72 ± 17.06 vs. 96.83 ± 11.25, p=0.01). Level of zinc reduced with aging. It seems that zinc levels are a risk factor for recurrent urinary tract infections. Yousefi, et al., [21] evaluated the therapeutic role of zinc in urinary tract infections. In a clinical trial study, they concluded that zinc supplementation has a significant effect in ameliorating severe dysuria and frequency of urinary in UTI. When the abdominal pain is present, usage of this drug is not recommended.
Results of a recent national study in Iran (2020) upon association of vitamin D, A and zinc deficiencies with stunting in toddlers (mean age=19.2 ± 8.4 months) published [25].
One of the limitations of the study was the low number of confirmed sepsis cases after complete evaluation.
Based on the current study, a significant percentage of septic young children (<5 years old) were deficient for zinc (57.6%) and vitamin A (42.4%) but none of controls. Low levels of vitamin A in our children (based on previous studies), although not a significant role in the development of limited bacterial infections (respiratory, gastrointestinal and urinary). But it seems that the deficiency of both zinc and vitamin A in young studied children predispose them to a severe form of bacterial infections and increases the risk of sepsis and mortality rate. So adding vitamin A and zinc to children's diets will reduce sepsis risk.
This article is a part of the doctoral dissertation entitled "Comparison of serum levels of vitamin A and zinc in children with sepsis admitted to the pediatric ward compared to the healthy group" in 2016 with the code 9311165017, which is supported by Iran University of Medical Sciences and Research Center for Infectious Diseases of Children of Rasoul Akram complex has been conducted.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref]
[Crossref] [Google Scholar] [PubMed]
[PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Noorbaksh S, Yusefi A, Sayahfar S, Ashouri S (2024) Vitamin A and Zinc Deficiencies in Developing of Childhood Sepsis: A Case-control Study in Tehran, Iran. Adv Pediatr Res. 11:071.
Received: 07-Feb-2024, Manuscript No. LDAPR-23-28271; Editor assigned: 09-Feb-2024, Pre QC No. LDAPR-23-28271 (PQ); Reviewed: 23-Feb-2024, QC No. LDAPR-23-28271; Revised: 01-Mar-2024, Manuscript No. LDAPR-23-28271 (R); Published: 08-Mar-2024 , DOI: 10.35248/2385-4529.24.11.071
Copyright: © 2024 Noorbaksh S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.