Journal of Pharmaceutical Care & Health Systems
Open Access
ISSN: 2376-0419
ISSN: 2376-0419
Review - (2020)Volume 7, Issue 2
Vaccine vial monitor (VVM) stands out amongst all the temperature monitoring devices and tools, as the only one that has shaped the course of vaccine management practices as well as the vaccine cold chain. Some critical vaccine management approaches that are practiced today, such as multi-dose vial policy, and controlled temperature chain have only been made possible with the help of VVM, and others such as rotating stocks and dispatches have been made more effective. Using the Albania immunization programme as a case, this paper reviews the vaccine management practices from the VVM perspective.
Vaccine Vial Monitor (VVM), Vaccine management, Multi-dose vial policy; Controlled temperature chain
Vaccines are biological medicinal products that are time and temperature-sensitive and must be stored and transported at controlled temperatures [1,2]. With the increasing number of vaccines entering the market, the supply chain today has become more complex requiring more effective and proficient operations. To warrant the quality of vaccines, manufacturers should meet the norms of international standards as well as their equivalence across manufacturers. However, consideration of this development, approval, and manufacturing process is not enough for ensuring quality throughout the lifecycle of a product, because vaccines spend substantial amount of time in countries at storage facilities, as well as in transport between warehouses, at hospitals, pharmacies, health centres and even within the houses of end-users. Product quality must be maintained until it is consumed. Vaccine management processes are designed to ensure product quality and comprise an integrated system of equipment, procedures, records, and activities [3,4]. Effective vaccine management requires that personnel handling vaccines should have the necessary knowledge and skills to perform their jobs effectively. Vaccine vial monitor (VVM) stands out amongst all the temperature monitoring devices and tools, as the only one that has shaped the course of vaccine management practices as well as the vaccine cold chain. Some critical approaches we have today in vaccine management, such as multi-dose vial policy, controlled temperature chain have only been made possible with the help of VVM, and others such as rotating stocks and dispatches have been made more effective [5,6]. This paper is prepared as a review of vaccine management practices from the VVM perspective, illustrating it with VVM based vaccine management practices in Albania immunization programme as a case study.
Albania immunization programme
Despite many challenges related to the health care system, social, and economic changes and migration, Albania has maintained a relatively well-performing immunization system with relatively high immunization coverage [7]. The National Immunization Program (NIP) is located within the Institute of Public Health (IPH) and is responsible for planning, forecasting, management and implementation of the nationwide immunization activities. The Directorate of Public Health (DPH) at the Ministry of Health (MOH) oversees public health district administration and the implementation of all public health programs in the country. The Albanian Health Insurance Fund (HIF) is responsible for health care facilities and employs health care workers, including vaccinators. District Public Health Directorates (DPHD) with their epidemiologic services provide methodological guidance, monitoring, supervision and assessment of the immunization services delivered by primary health care and maternity care facilities. They are also responsible for the planning and distribution of vaccines in their district, the surveillance of communicable diseases, epidemiological investigation of infectious diseases, outbreak response, and other activities related to infectious diseases at the district level.
Primary health care centers offer immunization services at the point of care. Vaccination is provided through a wide network of 2,282 baby and child services in urban health centers (including maternity hospitals) and in health posts in rural areas. Consultant pediatricians and family doctors are responsible for immunization services in urban and rural areas respectively. Dedicated vaccination staff exist at the municipality level and in the health centers. Staff positioned in village health posts have a broader set of tasks including vaccination. The organizational structure of the immunization system is illustrated in Figure 1.
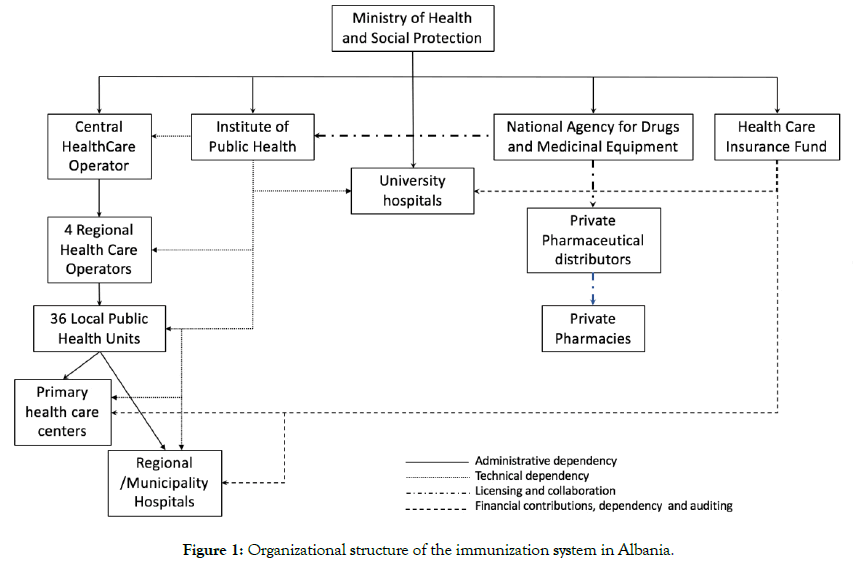
Figure 1: Organizational structure of the immunization system in Albania.
The immunization budget is a specific programme within the government budget and is managed by the IPH. The budget covers all vaccines, cold chain equipment, and injection safety supplies. A national immunization technical advisory group of experts (NITAG), establishedin 2015, provides guidance on the introduction of new vaccines, their effectiveness, impact, and safety based on the best evidence and current good practices and expert opinions.
Gavi the Vaccine Alliance provided support to the Albanian government for monovalent hepatitis B vaccine from 2001 to 2005 and Haemophilus influenzae type B (in pentavalent formulation) in 2008 for introduction in January 2009 for the period of 2009-2013 [8-10]. Following its transition from Gavi support and resuming full ownership of its immunization programme in 2011, Albania introduced pneumococcal vaccine at its own expense, making it a success story for the Gavi model of financial sustainability. Following a cost effectiveness study, Albania also has introduced the Rotavirus vaccine to its programme.
Between 2010 and 2012, the Project Optimize, a five-year partnership between the World Health Organization (WHO) and PATH collaborated with the IPH in Albania to demonstrate innovations in the supply chain that can help to meet the demands of an increasingly large and costly portfolio of vaccines [11]. One of the main objectivesof the project was to build an integrated Immunization Information System (IIS). Optimize project helped development of the IIS and IPH has implemented a web-based immunization registry [12]. The IISfacilitates recordsof personal immunization data and manages vaccine stocks and helps to identify cold chain gaps. The system is also used to register and follow adverse events following immunization. Health staff use the ISS to schedule immunization sessions as well. The system prioritizes the use of vaccines based on the VVM status and earliest-expiry-firstout (EEFO) principle during stock management process.
Nowadays IIS is owned by IPH and a lot of work has been done to upgrade and scale it up by including adult immunization and influenza vaccination and immunization campaigns.
The latest Effective Vaccine Management (EVM) assessment was conducted by an independent external team in Albania in 2012 (Table 1) [13]. Central and district level stores performance was over 80% in all criteria, while except maintenance and stock management, service level health facilities also scored above 80%. Vaccine management operations in the country were found to be effective based on the 80% EVM standard (Figure 2).
Table 1: Effective Vaccine Management (EVM) initiative [13].
| Launched by World Health Organization (WHO), the EVM initiative provides materials and tools needed to monitor and assess vaccine supply chains and help countries to improve their supply chain performance. |
| The EVM assessment looks into nine major areas: arrival procedures, temperature monitoring, storage capacity, buildings/equipment/transport, maintenance, stock management, distribution, vaccine management, and management information systems/supportive functions. A representative sample of sites is selected at level of the supply chain, and each of the nine EVM criteria is assessed at each level by observation, inspection of infrastructure and records, and by interview of staff. An area of vaccine management is considered “effective” if its criterion score is equal or greater than 80%, the EVM standard. |
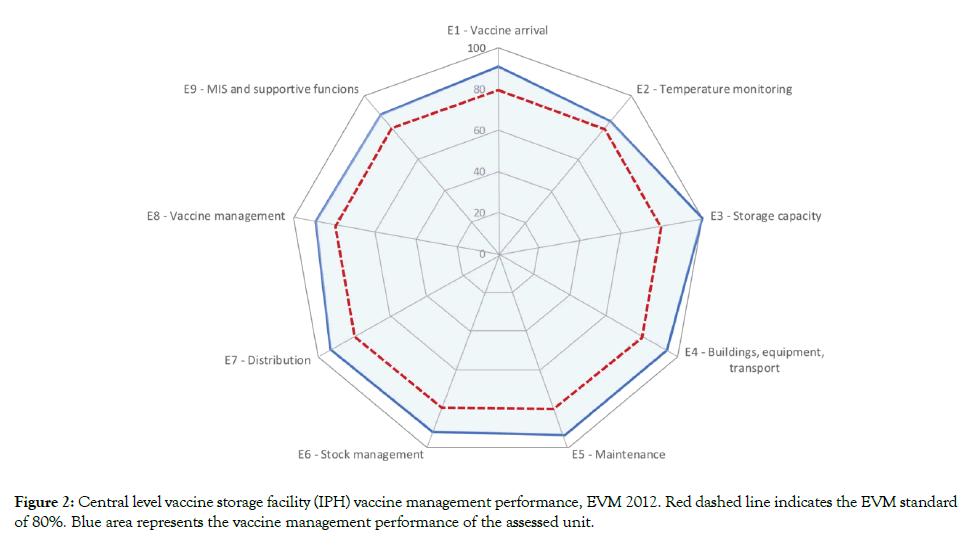
Figure 2: Central level vaccine storage facility (IPH) vaccine management performance, EVM 2012. Red dashed line indicates the EVM standard of 80%. Blue area represents the vaccine management performance of the assessed unit.
The immunization guideline published by the IPH, describes the immunization programme in detail and provides principles of EEFO, VVM use and multi-dose vial policy (MDVP). In 2010, following an intervention temperature monitoring study using 30- day electronic refrigerator temperature loggers, Albania introduced the WHO recommended 30-day electronic refrigerator temperature loggers for all immunization service points refrigerators. Temperature control record sheets were also modified to match with this introduction.
With the introduction of VVM and its expansion onto all vaccines globally, Albania continuously received vaccines with VVMs. Following its transitioning from Gavi, Albania continued to include VVMs among the minimum requirements for purchase of vaccines.
Although the services are improved, power outages still occur at different levels in the country. The World Bank reports an average of 4.1 power outages in a typical month (number) for 2013 [14]. In this regard, VVM is a critical tool for the health staff for making informed decisions in the case of power outages.
MDVP with the help of VVM is applied up to 28 days period following opening of multi-dose vials in Albania.
Vaccine Vial Monitor (VVM)
A vaccine vial monitor is a chemical time and temperature integrator label containing an active surface with heat-sensitive chemical in the shape of a square and a reference ring around. The VVM is permanently attachedto the label of the vaccine vial or on the flip-off top of the vial or on the neck on an ampoule and registers cumulative heat exposure over time. The color change taking place in the active surface of VVM is based on solid-state polymerization. The reaction is irreversible, and the rate of the color change increases with the temperature [15].
At the start point, the active square surface of the VVM is light in color compared to its reference ring and darkens by combination of time and temperature. The vaccine is usable since the inner square remains lighter than the outer circle. The color of the active surface reaches the same color of the reference ring when the temperature and/or the duration of heat exposure reach a level that is likely to degrade the vaccine beyond acceptable limit. This point is considered as discard point and from this point onwards (which active surface of VVM continues to darken as heat exposure continues and becomes darker than the outer circle) vaccine is recommended not to be used and discarded (Figure 3).
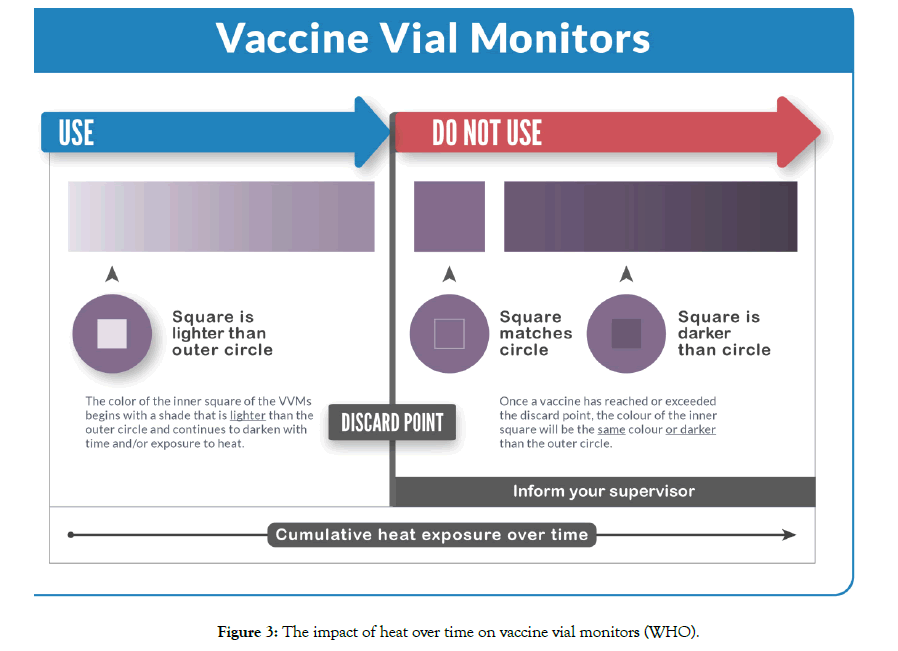
Figure 3: The impact of heat over time on vaccine vial monitors (WHO).
Based on their stability characteristics, vaccines are categorized in following groups of corresponding VVMs (Table 2) [16]. VVM performance specifications and their verification protocols are defined by the WHO Performance, Quality, and Safety (PQS) project.
Table 2: VVM reaction rates by type.
| Type (vaccines) | Maximum time to end point at 37°C | Maximum time to end point at 25°C | Maximum time to end point at 5°C | Time to end point at 5°C |
|---|---|---|---|---|
| VVM30: High stability | 30 days | 193 days | NA* | ≥4 years |
| VVM14: Medium stability | 14 days | 90 days | NA* | ≥3 years |
| VVM11: Intermediate stability | 11 days | 71 days | NA* | ≥2.5 years |
| VVM7: Moderate stability | 7 days | 45 days | NA* | ≥2 years |
| VVM2: Least stable | 2 days | NA* | 225 days | NA* |
| Type (vaccines) | Maximum time to end point at 55°C | Maximum time to end point at 45°C | Maximum time to end point at 37°C | Time to end point at 25°C |
| VVM250: Very high stability | 17 days | 73 days | 250 days** | ≥900 days |
*VVM (Arrhenius) reaction rates determined at two temperature points
**VVM (Arrhenius) reaction rates determined at 55°C and 45°C, the 37°C values are approximate
VVM is considered as one of the most significant recent innovations in public health. It is the only temperature monitoring device that is attached to a vial and present at all times from the time it’s manufactured until it is consumed. It provides a sensible and reliable signal to warn health workers whether the vaccine has been affected by heat and whether it can be used. The development of the concept of VVM goes way back to the 1970s when in two different locations and completely independently the idea was conceived [6]. It took close to 30 years to have the first commercial VVM on vaccines. Starting in 1996, VVMs started to appear on oral polio vaccines and following a WHO technical consultation meeting in 2002, the implementation of VVMs onto vaccines other than OPV started to accelerate. In February 2019, there were a total of 212 vaccine products from 36 vaccine manufacturers prequalified by the World Health Organization (WHO) andof these products, 196 of them werewith VVM. The UN market share for the vaccines with no VVM is extremely small (Figure 4).
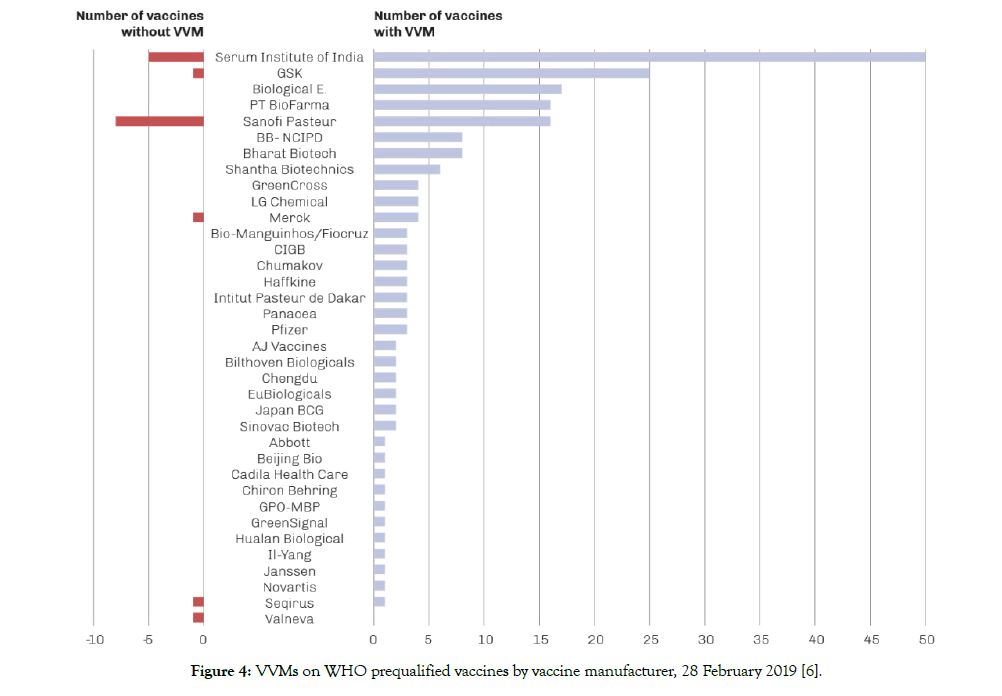
Figure 4: VVMs on WHO prequalified vaccines by vaccine manufacturer, 28 February 2019 [6].
VVM induced vaccine management policies
VVM is the only temperature monitoring device that dramatically changed vaccine management practices. It is the only device/tool in vaccine management that has a role in all levels of the vaccine supply chain starting with the acceptance of vaccines following an international shipment down to outreach sessions in remote areas in a country [15]. With its color reaction, VVM clearly indicates to health workers whether a vaccine can be used or not [17]. Some vaccine management approaches are fully based on the availability of VVM, while some others are enhanced with the presence of VVM.
Vaccine management functions from the VVM perspective
Operational and managerial vaccine management functions as suggested by the WHO are given in Table 3 [18]. Each and every function of the below listed vaccine management operations, relate to VVM.
Table 3: Operational and managerial vaccine management functions, WHO.
| Operational | Managerial |
|---|---|
|
|
These functions are incorporated into WHO effective vaccine management (EVM) assessment tool that assesses vaccine management functions through a systematic sampling in a country against nine high-level global criteria [19]:
1. Pre-shipment and arrival procedures ensure that every shipment from the vaccine manufacturer reaches the receiving store in satisfactory condition and with correct paperwork.
2. All vaccines and diluents are stored and distributed within WHO-recommended temperature ranges.
3. Cold storage, dry storage and transport capacity is sufficient to accommodate all vaccines and supplies needed for the programme.
4. Buildings, cold chain equipment and transport systems enable the vaccine and consumables supply chain to function effectively.
5. Maintenance of buildings, cold chain equipment and vehicles is satisfactory.
6. Stock management systems and procedures are effective.
7. Distribution between each level in the supply chain is effective.
8. Appropriate vaccine management policies are adopted and implemented.
9. Information systems and supportive management functions are satisfactory.
In this review, we will use the path vaccines follow in a country, starting with the procurement of vaccines to the outreach session at the periphery.
Procurement of vaccines
WHO and UNICEF calls upon all self-procuring Member States to include the VVM among the minimum requirements for vaccine purchase agreements [20]. Although there is no specific policy document, Albania as a self-procuring country requires VVM in all vaccines purchased, reflected in tender documents among the minimum requirements for vaccines, based on the national immunization guideline.
International arrival of vaccines
The decision to accept or reject an international vaccine shipment is based on the readings of electronic shipping indicators. The status of coolants and VVM serves as additional streams to support the mainstream decision that is based on electronic shipping indicators. Electronic shipping indicators are designed with the purpose of monitoring temperatures during transit. VVM is not a transit indicator, however, upon arrival VVM status should be checked and recorded. Albania follows WHO packaging and shipping guidelines in demanding temperature monitoring devices in international shipments and uses vaccine arrival report (VAR) to document the status of shipments on arrival [21-23]. Acceptance and rejection conditions that are in use in Albania are illustrated in the decision tree given in Figure 5.
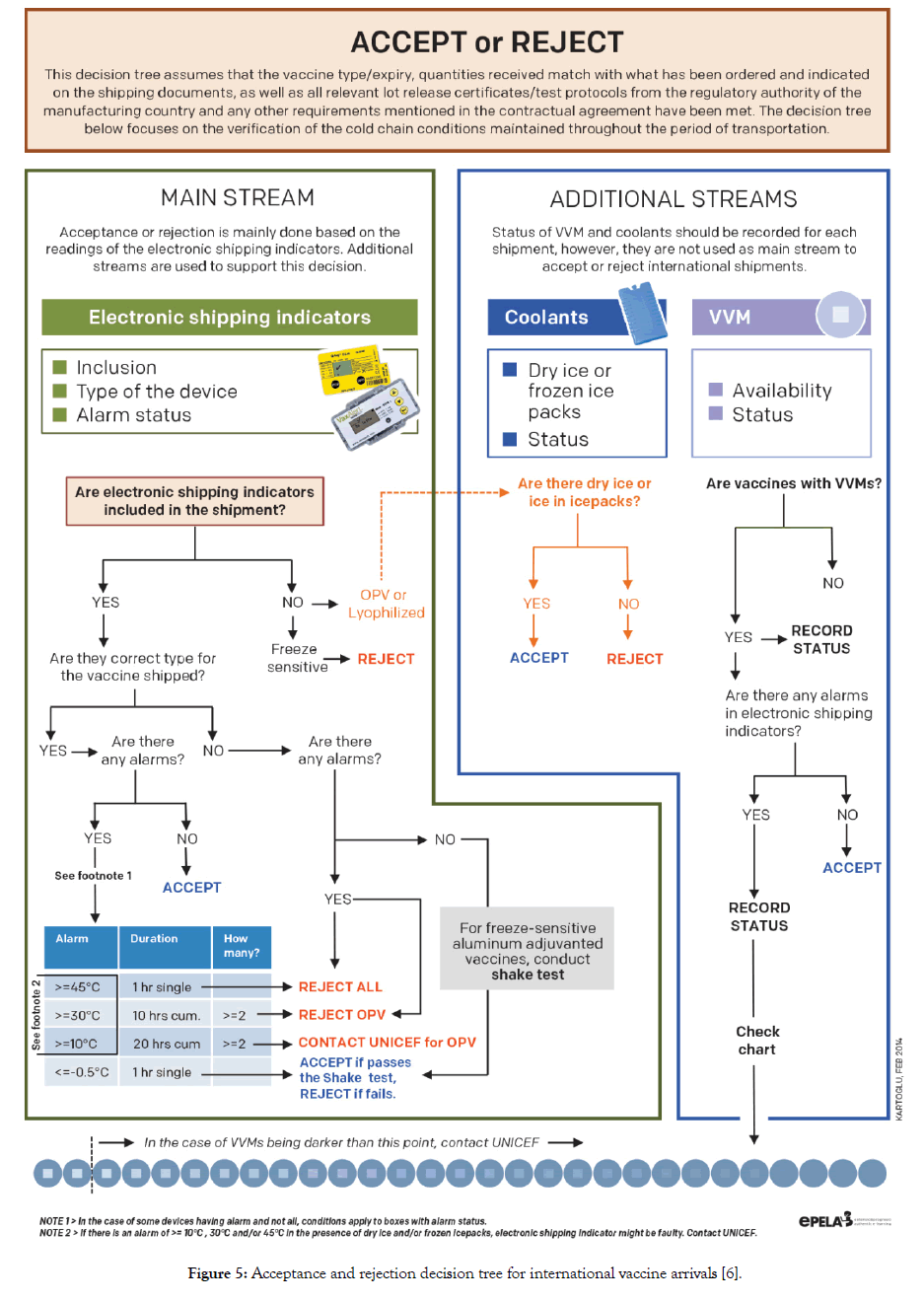
Figure 5: Acceptance and rejection decision tree for international vaccine arrivals [6].
Stock management
All biologicals products including vaccines and diluents have expiry dates marking the time after which they must not be used. In order to use products on time before they reach their expiry date, a stock management system must be designed allowing ample time for products to pass through the supply chain to lower levels.
When it comes to recording VVM status in stock records and dispatches, usable and not-usable type classification would be of little value since different shades of “usable” VVM will dictate which vials to be used both for dispatches and for immunization sessions. Although VVM reaction is continuous, for such practical purposes, 1-2-3-4 staging is used by countries. Albania also uses 4-level staging in stock management records. In this sense, such staging helps to give priority to vaccines with darker VVM (stage 2) compared to lighter ones (stage 1) in use and distribution (Figure 6).

Figure 6: Four staging in VVM, Albania.
The electronic immunization registry in Albania contains the expiry date as well as the VVM status of the vaccine that are in stock. As for VVM status, when stage 2 is indicated, the stage 2 VVM containing vaccines automatically go to the top of the list regardless of their expiry date to facilitate their dispatch/use first compared to vaccines with VVM stage 1. In manual stock records VVM status is also recorded both on arrival and the time of dispatch/use.
Using VVM as the smart expiry date
Previously, first-in-first-out (FIFO) inventory management principle was used universally. FIFO meant that the oldest stocks should be out first. Today, the earliest-expiry-first-out (EEFO) principle is practiced in the consideration that the oldest stock may not have the shortest expiry date. VVM is the only tool that may overrule the expiry date and brings an exception [23]. Let’s illustrate this with two different batches of the same vaccine with 12- and 6-month expiry remaining. EEFO principle would suggest that the batch with 6-month expiry should be dispatched first. This would be correct if there are no VVMs attached. Let’s assume that the vial with 12-month expiry has a darker VVM compared to the vial with 6-month expiry presenting a risk that if not dispatched, the darker VVM may reach its discard point before the next dispatch date. In this sense, the vial with a darker VVM will be needed to be dispatched first regardless of its expiry date (Figure 7) [6].
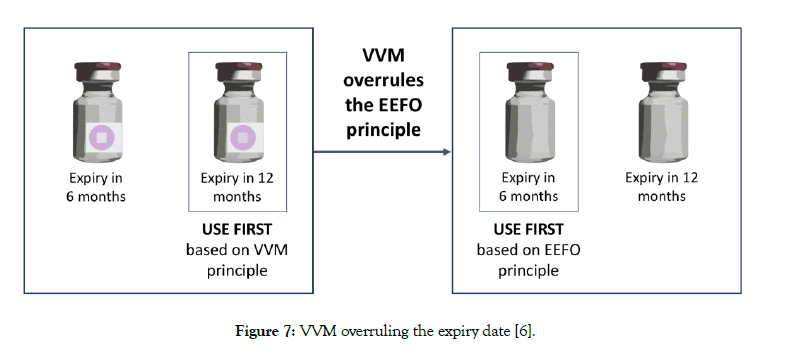
Figure 7: VVM overruling the expiry date [6].
Managing stocks with the help of VVM
The status of VVM should appear in relevant sections of all electronic and/or manual stock management forms recording the movements of vaccine products [24]. With the Project Optimize, Albania has introduced an online IIS. The system serves as a stock management tool to manage orders, stocks, and dispatches as well as to estimate orders, target children and control their vaccination records. The system has been currently upgraded and is in full use only in one municipality (the remaining ones are continuing to use a paper system with plans to be upgraded to online ISS). The VVM status is incorporated into the system as one of the main dominators in decision making for vaccine dispatches. When stage 2 VVM is entered for a particular batch, regardless of its expiry, batch with the stage 2 VVM is placed above the stage 1 VVM indicated vaccines. This allows responsible staff to dispatch the vaccines with darker VVMs first (Figure 8).
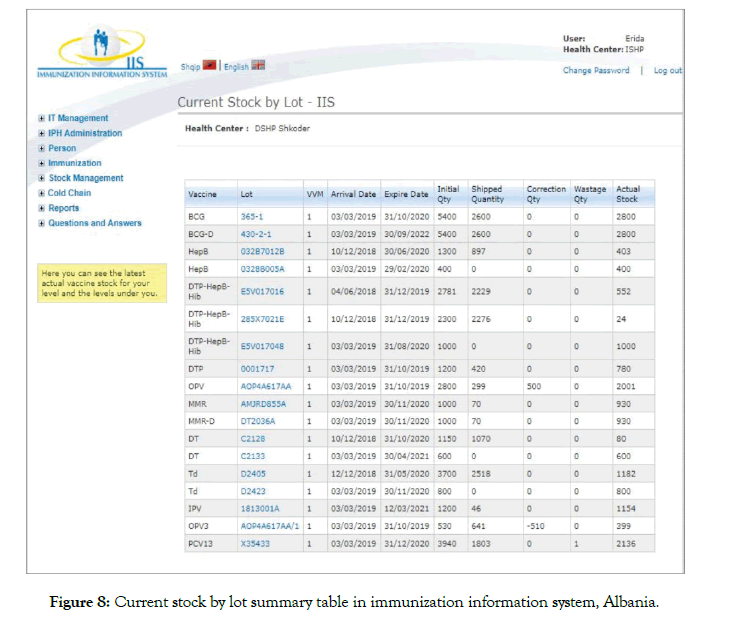
Figure 8: Current stock by lot summary table in immunization information system, Albania.
VVMs ruling the dispatches
In principle, five levels of control are suggested in deciding which vaccines to be dispatched [6]:
• VVM availability
• Number of batches
• VVM status and expiry
• Supply period and expiry
• Quantity to be dispatched
Albania immunization programme follows the algorithm illustrated in Figure 9 in dispatching vaccines from the storage facilities to lower levels.
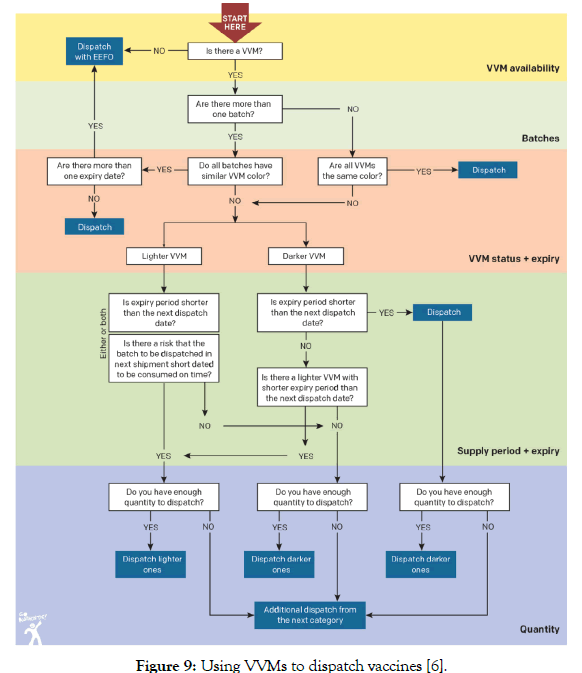
Figure 9: Using VVMs to dispatch vaccines [6].
VVMs pinpointing cold chain problems
VVM is a cumulative time and temperature integrator. Although VVM is not designed to monitor storage facilities and transport, it can take an active role in pinpointing problems related to maintenance of the cold chain.
As explained in Table 2, there are six types of VVMs matching different stability characteristics of vaccines. Naturally, reaction rates of these VVMs would be different even if they are exposed to the same temperatures [6]. For example, if a vaccine with a VVM7 is kept at 5°C for a year, the VVM would lose 35% of its life. On the contrary, a VVM30 would lose only 8% of its life under the same conditions.
Even within the recommended 2°C to 8°C temperature range, if the average temperature is closer to 8°C, the VVM reaction rate would be faster and naturally result in darker VVMs. Figure 10 provides additional information on the percentage of remaining shelf life within the recommended temperature range for a VVM7 product stored for about a year.
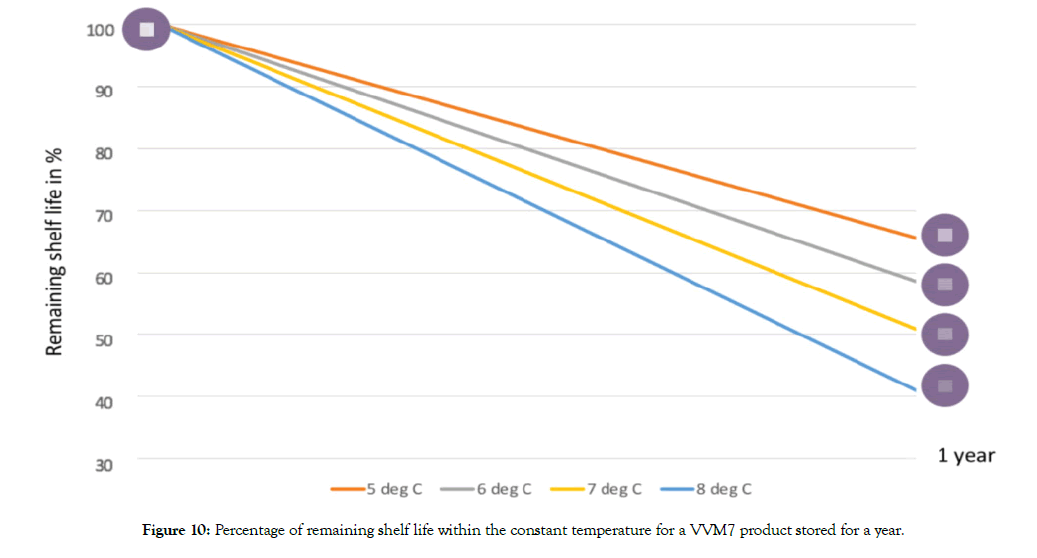
Figure 10: Percentage of remaining shelf life within the constant temperature for a VVM7 product stored for a year.
This information becomes critical when it comes to positioning the vaccines with VVM7 in the storage area. When VVM7 containing vaccines are not kept in the coolest area of the store, meaning that relatively temperature might be skewed towards the higher end of the recommended 2-8°C, they will become darker much faster. IPV used in Albania is with VVM7 and staffs are advised to keep the storage facilities at the mid-point of the recommended temperature range (5°C) and store the vaccines in the coolest part of the store. When there is an alarm
30-day electronic refrigerator loggersare the preferred temperature monitoring devices for health centre refrigerators as recommended by the WHO. Currently, there are four devices under this category that are prequalified by the WHO PQS. Albania immunization programme uses Fridge-tag2 (WHO PQS code E006/020) in the health centre refrigerators [25].
In the case of high alarm, both VVM status and position on the vial (as visual cue) are used for decision making. In the case of an alarm, Albania follows the algorithm given in Figure 11.
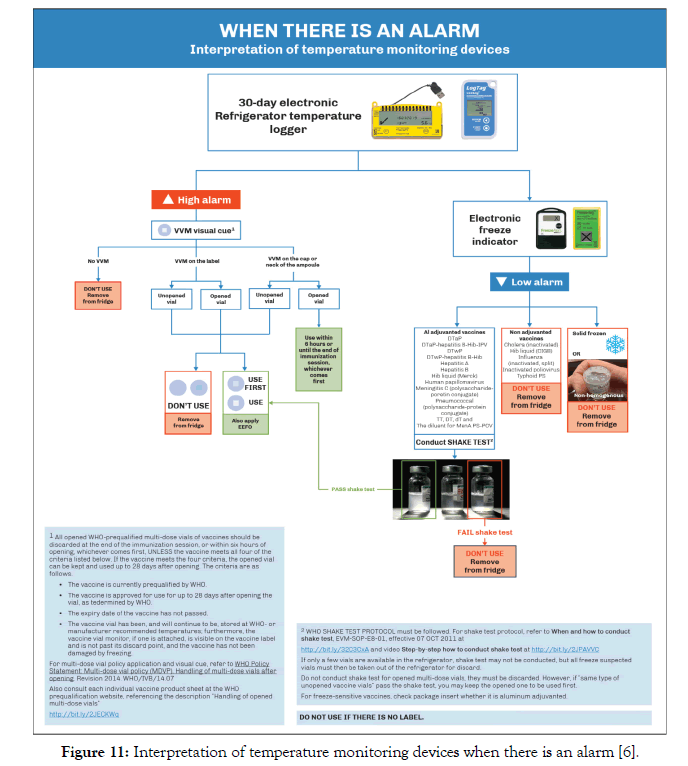
Figure 11: Interpretation of temperature monitoring devices when there is an alarm [6].
Multi-dose vial policy and VVM
The concept of using opened multi-dose vials in consequent immunization sessions was first introduced in 1995 as “open-vial policy” [26]. The policy was revised last time in 2014 and now is known as “multi-dose vial policy (MDVP)” [27]. MDVP lists conditions that are applicable for using opened multi-dose vials in subsequent immunization sessions. Not discarding and keeping the opened multi-dose vials for subsequent immunization sessions greatly reduces the vaccine wastage.
MDVP lists four conditions which if a vaccine meets all four conditions the opened multi-doe vial can be kept and used for up to 28 days after opening, otherwise all opened WHO-prequalified multi-dose vials of vaccines should be discarded at the end of the immunization session, or within six hours, whichever comes first. The criteria are as follows [27]:
1. The vaccine is currently prequalified by WHO.
2. The vaccine is approved for use for up to 28 days after opening the vial, as determined by WHO.
3. The expiry date of the vaccine has not passed.
4. The vaccine vial has been, and will continue to be, stored at WHO- or manufacturer recommended temperatures; furthermore, the vaccine vial monitor, if one is attached, is visible on the vaccine label and is not past its discard point, and the vaccine has not been damaged by freezing.
Before the 2014 revision of the MDVP, vaccines were divided into two categories, opened multi-dose liquid vaccines under certain conditions to be kept for use in subsequent immunization sessions while lyophilized vaccines were advised to be discarded at the end of the session or within six hours whichever comes first. At the time of the 2014 revision, it was not possible to fit all WHO prequalified vaccines in these two categories since some of them did not meet all defined standards applicable for the MDVP. In this sense, VVM was brought in as a visual cue to decide whether an open multi-dose vial of a vaccine can be used in subsequent immunization sessions or not. This was defined both in 2014 revision of the MDVP and in WHO PQS VVM product specifications (Table 4) [17]. All health workers in Albania are trained on the revised MDVP and the initiative is supported with new posters as reminders (Figure 12). One other protocol that is used in Albania immunization programme is to write down the date of the opening the vial with an indelible ink onto the label. By doing this, staff keep track of 28 days from the opening (Figure 13).
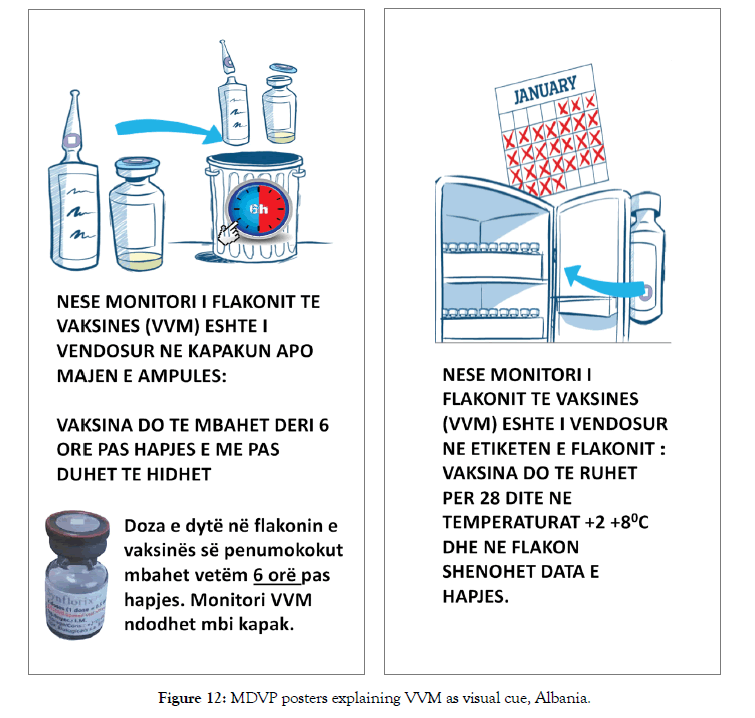
Figure 12: MDVP posters explaining VVM as visual cue, Albania.
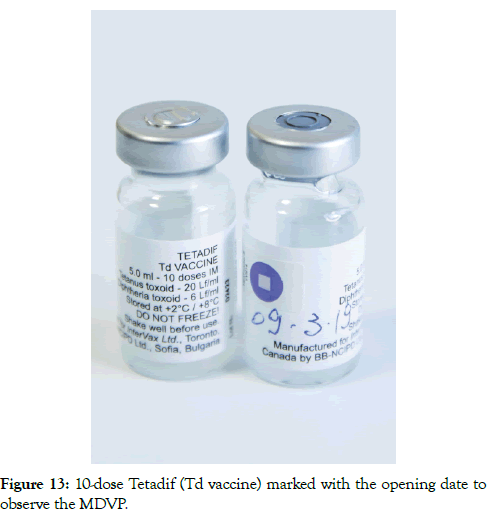
Figure 13: 10-dose Tetadif (Td vaccine) marked with the opening date to observe the MDVP.
Table 4: Integrity and location of VVMs [17].
| The location of the VVM on the vial depends upon whether the vaccine must be discarded at the end of the immunization session in which it is opened, or whether any remaining contents in an opened vial can be retained for use in subsequent sessions. The following cases apply: |
| For multi-dose vials containing a vaccine that can be used in subsequent sessions: Regardless of the vaccine presentation (liquid, freeze-dried or two vial combinations of liquid and freeze-dried), the VVM must be permanently attached to the label of the vaccine vial and must remain readily observable before, during, and after use, until the entire contents of the vial have been used. |
| For vaccines that must be discarded at the end of the session or within 6 hours, whichever comes first: The VVM must be attached to the vaccine vial or ampule and must remain readily observable until the vial or ampule is opened, but not observable after opening. In order to achieve this requirement, the VVM must be located on the flip-off top of a vial or on the neck of an ampule. |
| On a product by product basis, WHO will advise both the vaccine and the VVM manufacturer where the VVM is to be located. Locating the VVM on the bottom of a vial or ampule is never acceptable – it must always be in a visible location. |
From WHO/PQS VVM specifications (WHO/PQS/E006/IN05.3)
Controlled Temperature Chain (CTC)
There are examples of vaccine products that the product inserts states that the vaccine may be stored and used for limited periods above 8°C. However, for a health worker in the field, this information cannot be utilized since the health worker does not have any means of checking whether the vaccine was ever kept above 8°C and for how long to decide whether there is still room to keep vaccines at higher temperatures. VVM is the only tool that could help in such situations since it indicates whether the vaccine vial has been exposed to temperatures throughout the supply chain that could affect it. The innovative CTC approach as designed by WHO, allows certain vaccines to be exposed to ambient temperatures not to exceed +40°C for a defined period immediately before the administration and only once [28-30].
MenAfriVac® was the first vaccine to receive full licensure and WHO prequalification for use in a CTC in October 2012. This was followed by pneumococcal conjugate vaccine/PCV, Prevnar 13®, produced by Pfizer, in early 2015. However, the labelling has since removed any CTC indications. Human papillomavirus (HPV) vaccine, Gardasil4®, produced by Merck received full licensure and who prequalification for CTC use in mid-2016 [6]. Albania has not yet introduced any of the CTC approved vaccines into its immunization programme.
Removing ice from in-country transport of vaccines
Heat exposure has cumulative impact on all vaccines, however, freezing impact on freeze-sensitive vaccines is a “single event” meaning that if frozen, the protective properties of the vaccine is irreversibly lost [31]. Freezing occurs at all levels of the vaccine cold chain and in both industrialized and developing countries [32].
WHO recommends that icepacks should be fully conditioned to 0°C before being placed in the cold box for the transport of freeze-sensitive vaccines [33]. Conditioning of icepacks is done at room temperatures until the icepacks contain a mixture of ice and water. Naturally, it requires space (icepacks should be placed with space in between them not to create a very cold climate around that would seriously delay the conditioning). A typical large cold box requires 25 icepacks and conditioning of these packs to 0°C would require approximately an area of 1 m2 [34]. User compliance issues were reported from the field, and the practice was found to be impractical. Kartoglu and colleagues conducted a series of controlled laboratory and field studies on the use of cool water packs to prevent inadvertent freezing between 2002 and 2004 [35]. Following this study, WHO PQS introduced a new definition of “cool life” and incorporated it into the performance specifications and verification protocols for insulated containers [36,37]. Cool life was defined as “the empty container is stabilized at 43°C and loaded with cool-packs which have been stabilized at 5°C for a minimum of 24 hours. Cool life is measured from the moment when the container is closed, until the temperature of the warmest point inside the vaccine storage compartment first reaches 20°C, at a constant ambient temperature of 43°C.” WHO did not establish any standard for the cool life, but requires the performance data to be permanently displayed inside the lid.
Removing ice from in-country transport for all freeze-sensitive vaccines is simply the elimination of the known freezing hazard (that is ice) from the risk management perspective and is the most effective measure against freezing [38]. Removing ice from incountry transport is recommended only for vaccines with VVMs.
Albania removed icepacks from freeze-sensitive vaccine transportation in 2011. Today, all freeze-sensitive vaccines are transported using cool water packs. This resulted both saving staff time spent for conditioning, saving energy to freeze water packs, and most importantly eliminating the hazardous situations from in-country vaccine transportation.
As a visual signal, in addition to warning health workers for making informed decisions on whether to use the vaccine, VVM also facilitated the formulation of certain vaccine management policies, such as MDVP and CTC. VVM also made other vaccine management practices more effective such as vaccine dispatches, rotating stocks, and the use of cool water packs. Albania has a proven record of good vaccine management practices, scoring over 80% in all criteria in the most recent EVM assessment. Albania immunization programme is a good example of how VVM based vaccine management could be integrated into immunization programme. Here, the biggest challenge is the enhancing and expanding immunization staff capacity to effectively implement such an approach to realize the utmost benefits of VVM. Enhancing and expanding staff capacity is not taken as a onetime investment by the Albania immunization programme, rather routine refreshments, and supportive supervision are used as critical activities.
None to report.
This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors
Citation: Kartoglu U, Nelaj E, Preza I, Bino S (2020). Vaccine Vial Monitor Based Vaccine Management: An Albania Experience. J Pharma Care Health Sys 7: 1. doi: 10.35248/2376-0419.20.7.207
Received: 02-Feb-2020 Accepted: 06-Apr-2020 Published: 13-Apr-2020 , DOI: 10.35248/2376-0419.20.7.207
Copyright: �© 2019 Kartoglu U, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.