Andrology-Open Access
Open Access
ISSN: 2167-0250
ISSN: 2167-0250
Mini Article - (2024)Volume 13, Issue 5
This study mainly focused on the role of cholesterol dynamics in leydig cells to understanding testosterone deficiency in depression. Numerous studies have demonstrated a link between a higher risk of depression and low testosterone levels. Males with low testosterone frequently experience depressive symptoms and testosterone replacement therapy has been demonstrated to elevate mood in certain testosterone deficient individuals. Meanwhile, depression is frequently associated with hormonal imbalances, including testosterone deficiency. Recent research using chronic unexpected mild stress (CUMS) mice models has uncovered a significant connection between reduced testosterone levels and diminished cholesterol levels in Leydig cells, which are vital for testosterone production. This relationship highlights a significant intersection between mood disorders and metabolic processes.
Chronic unexpected mild stress; Cholesterol; Hypothalamic pituitary gonadal; Hypothalamic pituitary adrenal; Testosterone deficiency; Leydig cell
In the male testes, leydig cells are in charge of producing testosterone. Through a sequence of metabolic processes, they transform cholesterol into testosterone [1]. For male sexual function, proper testosterone levels must be maintained, which depends on these cells [2,3]. Depression can disrupt the Hypothalamic-Pituitary-Gonadal (HPG) axis, leading to altered levels of Luteinizing Hormone (LH) and Follicle Stimulating Hormone (FSH), which are critical for testosterone production and sperm production [4]. Cortisol, an end-product of the Hypothalamic-Pituitary-Adrenal (HPA) axis, is a stress hormone that plays a vital role in a wide range of physiological processes in the body. High concentrations of cortisol can directly result in apoptosis of rat Leydig cells in vivo and thereby decreasing testosterone synthesis [5]. Elevated cortisol levels can alter cholesterol metabolism, affecting its availability for conversion into testosterone in the Leydig cells, key enzymes such as P450scc (side-chain cleavage enzyme) and StAR (Steroidogenic Acute Regulatory Protein) were involved in this process [6].
Adequate cholesterol levels are vital for testosterone production in Leydig cells, any disruptions in cholesterol dynamics can impact testosterone synthesis. As a result, all tissues involved in steroid production, such as Leydig cells, have developed several paths for delivering cholesterol and an effective system for transporting cholesterol within cells. This ensures a continuous and sufficient supply of cholesterol. There are four main possible sources that could contribute to the hypothetical "Cholesterol Pool" required for steroidogenesis: The sources of cholesterol include 1) synthesis of new cholesterol molecules within the endoplasmic reticulum; 2) release of stored Cholesteryl Esters (CEs) from lipid droplets using cholesteryl ester hydrolase; 3) uptake of CEs derived from plasma lipoproteins through either Low-Density Lipoproteins (LDL) receptor-mediated endocytosis or (Scavenger receptor class B type I) SR-BI mediated selective uptake; and 4) acquisition of free cholesterol from the plasma membrane in certain cultured cell systems [7].
Leydig cells have the ability to produce cholesterol internally (de novo synthesis). The manufacture of cholesterol mainly takes place in the cell cytoplasm, specifically in the endoplasmic reticulum, using Acetyl Coenzyme A (Acetyl CoA) as a substrate in a sequence of enzymatic processes. Elevated cortisol levels can impact the functioning of enzymes that play a role in the production of cholesterol. For example, cortisol has the ability to suppress the activity of the enzyme HMG-CoA Reductase (HMGR), which plays a vital role in the synthesis of cholesterol from Acetyl-CoA [8]. This inhibition can reduce the availability of cholesterol for various physiological processes, including hormone synthesis. It is yet unknown, whether aberrant de novo cholesterol synthesis occurs in the leydig cells, and if so, how this affects testosterone levels in depressed men or animal models shown in Figure 1.
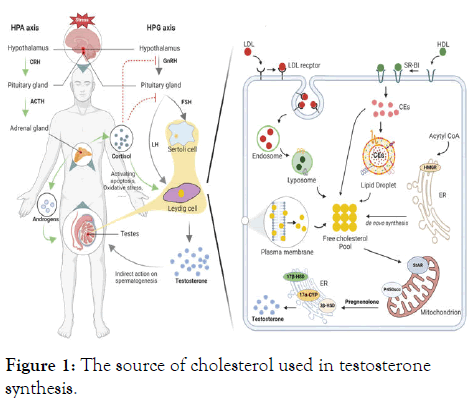
Figure 1: The source of cholesterol used in testosterone synthesis.
Apart from their natural production of cholesterol, leydig cells possess the ability to selectively uptake High-Density Lipoproteins (HDL) cholesterol via SR-BI-mediated selective transport of lipoprotein-derived cholesteryl esters and absorb LDL cholesterol through LDL-receptor-mediated endocytosis. Depression-associated cortisol elevation can influence the levels and function of lipoproteins such as LDL and HDL [9]. Cortisol has the ability to increase the liver's creation of LDL particles, which raises their levels in the blood [10]. The expression and functionality of SR-B1 can also be affected by cortisol [11]. The expression of LDLR and SR-BI is observed to be reduced in Leydig cells of the depression-like mice. This decrease may be closely associated with the decline in lipid droplet content in Leydig cells. However, the mechanism by which chronic unpredictable stress regulates the expression of LDLR and SR-BI requires further investigation. Studies investigating the role of Leydig cells in controlling the expression of SR-BI by means of autophagy, in order to maintain cholesterol balance, indicate that we can gain a better understanding of the precise mechanism by focusing on autophagy [12]. Altered ABCG1, a protein essential for transporting cholesterol from cells to HDL, can reduce the efficiency of cholesterol removal from tissues. Cortisol also exerts an effect on cholesterol efflux. Recently, it has been discovered that sphingomyelin plays a significant role in the process of ABCG1-mediated cholesterol efflux [13]. The presence of increased sphingomyelins in the testes and elevated levels of HDL-cholesterol in the blood indicate an improvement in ABCG1-mediated cholesterol efflux [3]. Further research will be conducted to investigate how depression affects cholesterol efflux.
CEs deposited in cytoplasmic lipid droplets is an additional source of cholesterol for Leydig cell steroidogenesis. Regardless of the origin of cholesterol, whether it is produced internally or obtained from external lipoproteins, cells that produce steroids tend to store cholesterol in the form of cholesterol ester in lipid droplets. This stored cholesterol serves as a reserve for the synthesis and maintenance of cell membranes, as well as a source of raw material for the production of testosterone in Leydig cells. Lipid droplets are composed of a central region containing neutral lipids (such as triacylglycerol and/or cholesteryl esters) and are enveloped by a single layer of polar lipids (such as cholesterol, phospholipids and fatty acids) that were initially derived from the ER. Perilipins and adipophilin, specifically coat the lipid droplets present in steroidogenic cells. Perilipins regulate the size, stability and metabolism of lipid droplets, which is essential for the generation of testosterone in Leydig cells [14]. Perilipins controls the process of lipolysis, it limits the entry of Hormone-Sensitive Lipase (HSL) and other lipases to the lipid droplets while conditions are normal, but allows lipase access to the droplets when conditions stimulate lipolysis. Hormone signals, such LH stimulate the release of cholesterol from lipid droplets. Stress causes a large change in LH levels, which may be related to the decreased lipid droplets in the leydig cells of mice that resemble depression [15]. In addition, hormones can regulate the degradation of lipid droplets through lysosomal processes, which prompts us to investigate the main mechanism responsible for reducing lipid droplets in Leydig cells of mice with depression-like symptoms.
Understanding testosterone insufficiency in the setting of depression requires an understanding of the complex interactions between cholesterol dynamics and testosterone synthesis in Leydig cells. Our investigation into how CUMS affects mice's Leydig cells indicates a complex link between decreased testosterone levels and changed lipid droplet dynamics and decreased cholesterol availability. The HPA and HPG axes are known to be disrupted by depression, which can result in hormonal abnormalities that have a major effect on testosterone synthesis. High cortisol levels, a defining feature of depression, hinder cholesterol synthesis by blocking important enzymes and changing lipoprotein metabolism. This perturbation impacts not only the synthesis of cholesterol within the body but also its absorption from outside sources. Furthermore, perilipins and other associated proteins regulate the storage and mobilization of cholesterol within lipid droplets, which is essential for preserving the availability of cholesterol for testosterone production. Those studies emphasize the significance of cholesterol dynamics in Leydig cells and show that the testosterone deficiency seen in depressive states can be caused by disturbances in cholesterol metabolism, whether through modified de novo synthesis, impaired lipoprotein uptake, or modifications in lipid droplet management. This link highlights the need for more investigation into the processes by which depression influences the production of testosterone and cholesterol. Comprehending these mechanisms may facilitate the development of innovative treatment approaches targeted at resolving hormonal dysregulation and mood disorders, thereby ameliorating the condition of those afflicted with depression and related low testosterone levels. Furthermore, certain research investigations have successfully identified isolated Mycobacterium neoaurum from the fecal samples of testosterone-deficient patients with depression and showed that this strain could degrade testosterone in vitro [16].
To sum up, including knowledge from Leydig cell cholesterol dynamics offers an invaluable viewpoint on the etiology of low testosterone in depression. Understanding these mechanisms will help us better target therapy to improve hormonal balance and reduce depression symptoms. This will eventually improve patient outcomes and further our understanding of the roles that endocrine and metabolic processes play in mood disorders.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Huang J, Peng Z, Song Z (2024). Understanding Testosterone Deficiency in Depression: Insights from Cholesterol Dynamics in Leydig Cells of CUMS Mice. Andrology. 13:327.
Received: 02-Aug-2024, Manuscript No. ANO-24-33789; Editor assigned: 05-Sep-2024, Pre QC No. ANO-24-33789 (PQ); Reviewed: 19-Sep-2024, QC No. ANO-24-33789; Revised: 26-Sep-2024, Manuscript No. ANO-24-33789 (R); Published: 03-Oct-2024 , DOI: 10.35248/2167-0250.24.13.327
Copyright: © 2024 Huang J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.