Journal of Infectious Diseases & Preventive Medicine
Open Access
ISSN: 2329-8731
ISSN: 2329-8731
Review Article - (2025)Volume 13, Issue 1
Truenat is a novel chip based test developed in India for TB diagnosis. Its use for pulmonary samples has been approved by WHO. Government of India has recently approved it for extrapulmonary cases. This review gives an insight on the results of various studies conducted on Truenat so far and its implications for future use.
Truenat tuberculosis micro-RTPCR; Pulmonary tuberculosis; Sensitivity; Specificity; Rifampicin resistance
Truenat MTB is a chip based, portable micro RT-PCR developed by Molbio Diagnostics (Bigtech labs), India. Its lower cost, better shelf life, an inbuilt rechargeable battery system and the fact that it does not require air-conditioned room make it a good alternative to CBNAAT.
Three variants of the test are present:
Truenat set up can be deployed at the lowest level of the healthcare pyramid, thus reducing the logistical delays that occur in transporting the sample to a higher centre. This allows for early detection of the Bacillus. Furthermore, there is processing of only a single sample in Truenat Uno which prevents cross contamination issues. As this is a portable platform, it can be used for active case finding programs at peripheral level as well.
Truenat procedure
The sample is first liquified to extract DNA, by using liquefaction buffer. Then the sample is mixed with the lysis buffer (Figure 1).
Figure 1: Sample of Truenat AutoPrep.
Then the sample prep device-Truenat AutoPrep V2 is used for extraction and purification of nucleic acid from the sample. The sample is put into the sample chamber of the cartridge and the cartridge is inserted into the AutoPrep device which extracts the DNA from thermal lysis of the cells and also washes out the PCR inhibitors. The extracted DNA is collected in the elute chamber of the cartridge. DNA extraction is done in ~20 minutes (Figures 2 and 3).
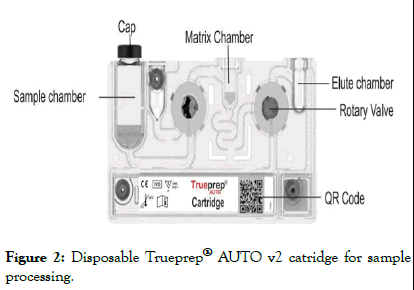
Figure 2: Disposable Trueprep® AUTO v2 catridge for sample processing.

Figure 3: Extracted DNA is collected in the elute chamber.
Then the sample is loaded into the Truenat chip, which is inserted into the micro PCR analyzer (Figures 4 and 5).
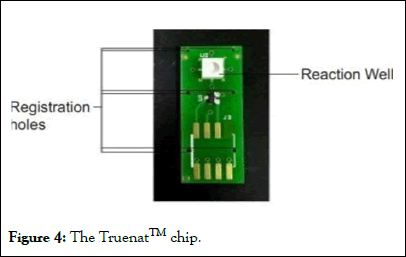
Figure 4: The TruenatTM chip.
Figure 5: Loading the sample on to the chip.
The PCR analyser comes in the three forms-UNO Dx, Duo and Quatro which can test one, two and four samples respectively, at a time. It runs 40 cycles of PCR in 35 minutes i.e., it displays result in ~40 minutes (Figure 6).

Figure 6: PCR analyser comes in the three forms-UNO Dx, Duo and Quatro.
The result screen also displays the Ct value and the colony forming units CFU/ml for positive specimen. In case the sample is positive, another test is done to detect Rifampicin resistance in over 60 minutes. Both the Trueprep and Truelab devices are portable, battery operated and can function in upto 40C temperature and 80% relative humidity.
The first study on Truenat was conducted at Hinduja Hospital and Medical Research Centre Mumbai by Nikam, et al. [1], in collaboration with Bigtec labs, Bangalore to evaluate the performance of Truenat in comparison with GeneXpert on sputum samples of pulmonary TB patients. The sensitivity of Truenat was calculated to be 94.7% whereas that of GeneXpert was 96%. In the sputum positive, culture positive samples, Xpert detected 100% of the cases, whereas Truenat detected 98.92% cases. High concordance was observed between Gene Xpert and Truenat. Out of 247 samples, 229 samples i.e., 92.7% showed identical results for both Xpert and Truenat MTB.
In yet another study by Nikam, et al. [2], in Hinduja Hospital, 226 sputum samples from suspected pulmonary tuberculosis patients were examined. Out of the 226 samples, 110 were smear positive, 141 were culture positive, 174 were Truenat positive and 176 samples were positive on in-house nested PCR. In house PCR and Truenat had higher sensitivity than microscopy and culture.
A study was conducted by Reena Anie Jose [3], in a tertiary care centre in Kerala in 2019. In this study extrapulmonary samples were tested by smear, culture and Truenat MTB Plus to assess the sensitivity, specificity, positive predictive value and negative predictive value of Truenat. Keeping culture as the gold standard, Truenat had the following results: Sensitivity-100%, specificity-95.1%, positive predictive value-44.4%, negative predictive value-100%, observed agreement-95.3%. When compared with microscopy, Truenat test had 100% sensitivity and 96.6% specificity and observed agreement of 96.6%.
A blinded, multicentric study was conducted across four sites in India in 2019-ICMR, AIIMS New Delhi, NITRD, New Delhi and National JALMA Institute for Leprosy and Other Mycobacterial Diseases, Agra [4]. The study was done on 2623 sputum samples on presumptive pulmonary TB cases in adult population.
The study concluded that Truenat was more precise in detecting MTB than Xpert. Moreover, operational feasibility was also assessed in this study which demonstrated that Truenat testing required minimal training and there were no equipment failures reported from any of the sites in the study period.
A prospective cross sectional study was conducted in Tamil Nadu, India by V. Mangayarkarasi, et al. [5]. The study had the following results (Table 1).
|
Culture positive |
Culture negative |
|
|
Truenat positive |
27 |
14 |
|
Truenat negative |
2 |
37 |
Table 1: Concordance results of Truenat and culture on pulmonary samples.
Truenat sensitivity and specificity-93.1% and 72.5% respectively (Table 2).
|
Culture positive |
Culture negative |
|
|
Truenat positive |
30 |
8 |
|
Truenat negative |
1 |
26 |
Table 2: Concordance results for Truenat and culture on extrapulmonary samples.
Truenat sensitivity and specificity-96.77% and 76.4% respectively.
Among the 145 samples included in this study, Truenat detected 54.48% cases while culture detected only 41.3% cases.
A study was conducted by Georghiou, et al. [6], in 2021 to validate Truenat MTB-RIF Dx accuracy for RIF resistance mutation detection. It was found that Truenat MTB-RIF Dx assay detected 13/15 of the RIF resistance mutations representing ~98.6% accuracy for global prevalence of RIFresistant strains, compared to 99.3% for the MTBDR plus assay. The Truenat MTB-RIF Dx assay detected all mutations except rpoB Q432K and S441L and the MTBDR plus assay detected all mutations except rpoB S441L. Hence the study concluded that Truenat MTB-RIF Dx had excellent performance for detection of rifampicin resistance detection.
Another prospective, multicentre study was conducted by Adam Penn-Nicholson, et al. [7], in 19 primary healthcare centres and 7 reference laboratories in India, Peru, Ethiopia and Papua New Guinea. Study subjects were adults with pulmonary TB. 1807 samples were assessed, out of which 24% were culture positive and 15% were rifampicin resistant. The study assessed Truenat- MTB, Truenat MTB-Plus and Truenat MTB-RIF Dx. The following result was obtained (Table 3).
|
Truenat MTB |
Truenat MTB Plus |
Truenat MTB-RIF Dx |
|
|
Sensitivity |
73% |
80% |
84% |
|
Specificity |
98% |
96% |
95% |
Table 3: Sensitivity and specificity of Truenat.
In this study 1542 samples were assessed with Truenat, Gene Xpert and culture and it was concluded that there was no significant difference in the performance of the two nucleic acid amplification techniques.
Another study was conducted by Abyot Meaza, et al. [8] in Ethiopia. It was a prospective study conducted between May 2019 and December 2020 and included 200 adults with pulmonary tuberculosis (Table 4).
|
Truenat MTB |
Truenat MTB Plus |
|
|
Sensitivity |
88% |
91% |
|
Specificity |
97.20% |
97.20% |
Table 4: Sensitivity and specificity of Truenat tests against reference standard.
The study also concluded that the diagnostic accuracy of Truenat tests was comparable to Xpert MTB-RIF.
In a retrospective study by Valsan, et al. [9], from Kerala, Truenat was compared with MGIT in pulmonary as well as extrapulmonary samples. In this study, 21 (17.6%) out of 119 pulmonary samples were positive by Truenat and 20 were positive by MGIT. The overall sensitivity and specificity of Truenat with MGIT was found to be 90% and 96% respectively.
This study had 342 extrapulmonary samples, out of which 64 were CSF samples. The overall positivity of Truenat in extrapulmonary samples was 11.9% (Table 5).
|
Truenat positive |
Truenat negative |
|
|
MGIT positive |
31 |
17 |
|
MGIT negative |
10 |
284 |
Table 5: Depicting concordance between Truenat and MGIT in extrapulmonary samples.
The study had 64 CSF samples, out of which only 2 (3.1%) were positive on Truenat. The overall positivity of Truenat in extrapulmonary samples was 3.1% (Table 6).
|
Truenat positive |
Truenat negative |
|
|
MGIT positive |
1 |
3 |
|
MGIT negative |
1 |
59 |
Table 6: Depicting concordance between Truenat and MGIT in CSF samples.
A study has been conducted in PGIMER Chandigarh comparing Truenat-MTB PLUS (TruPlus) with Gene Xpert ULTRA for CSF samples. TruPlus targets multicopy IS6110 along with single copy nrdZ gene for the detection of M. tuberculosis. It has LOD 30 CFU/ml in contrast to 100 CFU/ml in Truenat. The study included 108 (76 definite plus 32 probable adult TBM patients based on Marais criteria). Among these 108, 85 were positive on TruPlus and 73 were positive on ULTRA. There were 23 cases which were picked up only by TruPlus and 11 cases which were positive only in ULTRA. The study showed sensitivity of TruPlus to be 78.7%.
Another study has been conducted by Urwashi singh, et al. in suspect pulmonary tuberculosis pediatric patients from North India. This study included 612 sputum samples which were tested by smear, liquid culture by MGIT, Truenat and GeneXpert. This study showed sensitivity and specificity of Truenat against liquid culture to be 58.3% and 88.3%. Similarly sensitivity and specificity of GeneXpert against liquid culture was 53.6% and 91.9% (Table 7).
| Study | Population | Sample | Test | Sample size | Sensitivity | Gold Standard |
| Nikam, et al. | Adult | Pulmonary | Truenat | 274 | 94.70% | MGIT |
| RA Jose, et al. | Adult | Extrapulmonary | TruPlus | 248 | 100% | Culture |
| Mangayarkarasi, et al. | Adult | Pulmonary | Truenat | 80 | 93.10% | Culture |
| Extrapulmonary | 65 | 96.70% | ||||
| Nicholson, et al. | Adult | Pulmonary | Truenat | 1807 | 73% | ÃÂÂ |
| TruPlus | 80% | |||||
| Meaza, et al. | Adult | Pulmonary | ÃÂÂ | 200 | 88% | Culture |
| Valsan, et. | Adult | Pulmonary | Truenat | 119 | 90% | MGIT |
| Sharma, et al. | Adult | CSF | TruPlus | 108 | 78.7% | Definite plus probable TB |
| Singh, et al. | Pediatric | Pulmonary | Truenat | 612 | 58.3% | Liquid culture |
Table 7: Sensitivity of Truenat in various studies.
The molecular tests have high sensitivity and specificity against culture as gold standard, but these parameters decline when compared against clinical criteria, as seen in study by Sharma, et al. [10]. At times, RTPCR studies pick up the bacilli even when culture fails to do so. Hence, in cases of tuberculosis, it is of utmost importance to evaluate the patient fully in terms of clinical, radiological and biochemical parameters before ruling out the disease. The biochemical studies confirm the presence of the bacilli, but are of limited importance for ruling out the disease. The main reason for this is the paucibacillary nature of the specimen, inadequate specimen or inappropriate specimen (for example, BAL has higher yield than sputum for diagnosis of pulmonary tuberculosis).
As is evident in various studies, Truenat has comparable sensitivity and specificity to GeneXpert.
But when individual cases are studied, concordance between two studies ranges from 65% to 85%. The discrepancy in results may be due to the fact that these two tests target different genes for identification [11].
Truenat is a novel micro RT PCR test which has high potential for use in peripheral centres because of minimal set up and requirements. Molecular tests should not be used alone for evaluation of a suspected TB patient. Further studies are required to ascertain the cause of discrepancy in the results of various RTPCR tests.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Sharma S (2025) Truenat MTB-Role in diagnosis of Tuberculosis. Infect Dis Preve Med. 13:398.
Received: 30-Mar-2024, Manuscript No. JADPR-24-30555; Editor assigned: 01-Apr-2024, Pre QC No. JADPR-24-30555 (PQ); Reviewed: 15-Apr-2024, QC No. JADPR-24-30555; Revised: 07-Feb-2025, Manuscript No. JADPR-24-30555 (R); Published: 14-Feb-2025 , DOI: 10.35841/2329-8731.25.13.398
Copyright: © 2025 Sharma S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.