Andrology-Open Access
Open Access
ISSN: 2167-0250
ISSN: 2167-0250
Review Article - (2021)Volume 10, Issue 8
Background: Trans-sexualism is a disorder when an individual feels inconsistent with his/her assigned sex, and desires for a permanent transition to the opposite sex. It has for long been considered a whim of a disturbed mind. However, it may be a true biological disturbance that needs correction earlier better than later in the age of the person who shows the desire of transition to the opposite sex. Objectives: To highlight the issue of trans-sex, its incidence and prevalence, and its biological causes, to remove the confusion and the many wrong concepts about this medical problem. Methods: A comprehensive systematic search for the data, in the search engines using the key words, using a repeatable method; appraising the data (and the quality of the data) with filtration to exclude duplication and a synthesis of research data. Trans-sex individuals exhibit persistent and authentic differences from the cis-sex individuals. The early decision is better because of the psychological, social and job consequences of sex in the life of the person. Societies’ intolerance to trans-sex persons may be very challenging and can cause anxiety disorders, depression, and other psychologic disturbances. Conclusion: Trans-sex is a true biological disorder that needs proper diagnosis and management. Reaching a diagnosis in cases of trans-sex requires physicians to be oriented of the rare, but an existing, biological disturbance and how to approach for reaching a diagnosis of trans-sexualism. HY-antigen typing, brain imaging for the anatomical differences between male and female brains, and other investigations that are useful in such cases and are discussed in this review.
Trans-sexualism; Wrong concepts; HY-antigen; Investigations
FtM: Female to Male; GID: Gender Identity Disorder; LCW: Lobar Connectivity Weight; MtF: Male to Female, PCR: Polymerase Chain Reaction
Trans-sexualism is a disorder when an individual identify himself/herself as belonging to the opposite sex to which he/she was born with Hines [1]. It has for long been considered a whim of a disturbed mind. However, it may be a true biological disturbance that needs correction earlier better than later in the age of the person who shows the desire of transition to the opposite sex. Trans-sex individuals exhibit persistent and authentic differences from the cis-sex individuals (Burke et al. [2].This early decision is better because of the psychological, social and job consequences of sex in the life of the person. Societies’ intolerance to trans-sex persons may be very challenging and can sometimes cause anxiety disorders, depression, and other psychologic disturbances Winter et al. [3].
Aim
This review aims to shed light on the issue of trans-sex, and to bring the biological causes and true facts about the problem, that remove the doubt and the many wrong ideas about an existing and increasing biological disturbance, and on the other hand, there is no sufficient knowledge and understanding from the medical community and the whole society.
Methodology
A comprehensive systematic search for the data, in the search engines (PubMed, ScienceDirect, Medline, Scopus, Med know) using the key words (Trans-sexualism- Wrong concepts- Causes- Investigations), using a repeatable method; appraising the data (and the quality of the data) with filtration to exclude duplication and a synthesis of research data.
A systematic review of the literature published since inception till July 2021 was done. Relevant articles were determined based on the key words (Trans-sexualism- Wrong concepts- Causes- Investigations). Duplicate data were excluded. Research papers on 'Gender Dysphoria' were excluded too.
Only the biological trans-sex papers were included.
So, 87 studies were included from PubMed, Cochrane database, Science Direct and the web of knowledge, and 8970 studies on the trans-gender and pure social sex dysphoria with no biological trans-sex, were excluded.
Sex differentiation is of a very high health, scientific and societal importance and it reflects upon the human behavior, function and being. Sex disparities are very challenging problems with very limited, and may be, a wrong knowledge about, in medical communities. The issue of transsex people constitutes an overlooked aspect of current research Hilário et al. [4].
History of the trans-sex problem
In USA in 1917, Alan Hart, became the first female-to-male transsexual. She did hysterectomy and gonadectomy to relieve gender dysphoria. In Berlin in 1931, Dora Richter, was the first male to female by doing vaginoplasty. In Dresden during 1930-1931, Lili Elbe removed her original sex organs, and did four subsequent operations for a male-to-female, that included an unsuccessful uterine transplant, resulted in rejection and in her death Sherman et al. [5].
The Table 1 shows the incidence of trans-sex among males and females in some countries Davy et al. [6]. On the other hand, the prevalence of Gender Dysphoria (GD) varies internationally because of different cultural and societal norms, and the differences in definitions of DSD (Disorders of Sex Development). The prevalence in Australia is unknown as there is a paucity of studies in this area. New Zealand reported an estimated prevalence of 1 in 6000 in one study. However, researchers suggest that the prevalence is much higher than that reported and what was previously thought Marshall et al. [7] (Tables 1 and 2).
| % in Females | % in Males | Year | Publications | Country |
|---|---|---|---|---|
| 02:40.0 | 1 30.000 | 1994 | DSM-Iv | US |
| 10:48.2 | 10:48.2 | 1996 | Acta Psychiatrica Scandinavica | Sweden |
| 01:30.0 | 01:10.0 | 1997 | The Journal of Clinical Endocrinology and Metabolism | Netherland |
| 01:08.0 | 01:04.5 | 2007 | International Journal of Transgenderism | US |
| 01:22.7 | 01:03.6 | 2008 | Australian and New Zealand Journal of Psychiatry | New Zealand |
| 01:15.0 | 01:11.0 | 2016 | The Journal of Sexual Medicine | US |
Table 1: The incidence of trans-sex among males and females in some countries [6].
Genetic factors
HY-antigen: It is a minor histocompatibility antigen that presents in all tissues of normal males, and is coded for by a structural gene on the short arm of the Y chromosome; it is thought to promote the differentiation of indifferent gonads into testes, thus determining a male sex Kim et al. [8].
SRY gene: Mammalian sex determination depends on the development of gonads to ovaries or to testes. The testis development is being triggered by the expression of the transcription factor Sex-determining Region Y (SRY). SRY is a small, single-exon gene that is located in the male-specific region of the Y chromosome (She et al. [9]. Several epigenetic modifiers, transcription factors and kinases are implicated in regulating Sry gene transcription. The expression of Sry gene in the genital ridges typically results in their development into testes, whereas the absence or dysfunction of Sry leads the undifferentiated gonads to develop to ovaries Capel [10]. Brittany et al. stated that SRY and its target SOX9, are an established cause of 46 XY DSD, and the investigators believed that the genetic basis of many DSDs still remains unknown [11].
The androgen nuclear receptor variant 2 (NR3C4): Mutations in NR3C4 gene, located on the X chromosome at Xq11-12 and encoding the Androgen Receptor (AR), can cause DSD. It can also result in functional consequences like the Androgen Insensitivity Syndrome (AIS). The reduction in androgen or in androgen's signaling leads to a female character of a developing brain in a male embryo Ramos et al. [12].
Female-specific cytochrome P450 (CYP) 17-34 T>C: It is a gene located on the human chromosome 10 and is translated to the female sex hormones pregnenolone and progesterone. The loss of a female-specific CYP17 T-34C allele is associated with a Female-to-Male (FtM) transsexualism. It can be detected by multiplex PCR on a microarray system of buccal swabs samples Bentz et al. [13] (Table 2).
| Connection | T statistic | P value |
|---|---|---|
| LF-LF | 5.06 | <0.000001 |
| LF-LT | 5.06 | <0.000001 |
| LF-LP | 7.29 | <0.000001 |
| LT-LT | 7.15 | <0.000001 |
| LT-LP | 5.07 | <0.000001 |
| LT-LO | 5.95 | 0.000001 |
| LP-LP | 6.78 | <0.000001 |
| LP-LO | 4.03 | 0.000061 |
| LO-LO | 4.89 | <0.000001 |
| LF-RF | −4.74 | 0.0000024 |
| RF-RF | 5.63 | <0.000001 |
| RF-RT | 5.02 | <0.000001 |
| RF-RP | 7.39 | <0.000001 |
| RT-RT | 5.65 | <0.000001 |
| RT-RP | 4.77 | 0.000002 |
| RT-RO | 5.26 | <0.000001 |
| RP-RP | 5.83 | <0.000001 |
| RP-RO | 3.22 | 0.00013 |
| RO-RO | 3.78 | 0.00017 |
A positive T statistic indicates that the male group had higher value than the female group, and vice versa. F, frontal; L, left; O, occipital; P, parietal; R, right; T, temporal.
Table 2: Lobar Connectivity Weight (LCW) differences between men and women [31].
Other genetic aberrations and sex reversal: Human testis-specific enhancers are genes, that when duplicated or deleted, lead to 46-XX or 46- XY DSD (sex reversal), respectively, with no effect on other developmental processes that require SOX9 (i.e. absence of skeletal abnormalities or other features of campomelic dysplasia) García-Acero et al. [14]. SRY, SF1 and SOX9 initiation, upregulation and maintenance, are all necessary for the testis development and ultimately the human male development. These factors are determined by PCR analysis and the microarray mapping. On the other hand, the Steroid 5-Alpha Reductase (SRD5A2) Val89Leu Single Nucleotide Polymorphism (SRD5A2 Val89Leu SNP) involved in androgens metabolism is not associated with transsexualism, refuting SRD5A2 as a candidate gene of transsexualism Bentz et al. [15]. So, genetic variance and pleiotropy seem to affect sex-specific adaptation through changes in gene expression, linking local adaptation with the evolution of sex differences' Ramos et al. [12].
Hormonal factors
Prenatal androgen exposure: Congenital adrenal hyperplasia in persons with XX sex chromosomes leads to a masculinization in a female and a tendency to the same sex attraction (homosexuality). It is reported that at least 5.2% of these individuals develop serious gender dysphoria. The condition is reversible with anti-androgens medications Batista et al. [16].
The 5-alpha-reductase deficiency: The 5-alpha-reductase deficiency impairs the conversion of testosterone to dihydrotestosterone, decreasing the masculinization and male genitalia growth, making these individuals raised as females with a high percent of an FtM transition later in their life affected by pubertal hormonal surge and social factors. Scientists decided that the definition of masculine characteristics during puberty and the increased social status afforded to men are two possible motivations for a female-to-male transition Jabeen et al. [17].
Neurological factors
Transsexuals are reported to have the strong feeling, often from the early childhood onwards, that they belong to the opposite sex. The investigation of the differences between men and women are always of a great interest to neuroscientists. The structural and the functional aspects of the human brain show marked sex differences (Floris et al. [18].
These differences are macroscopic like the differences in the overall brain and lobar volumes, the gray/white matter ratio, and the corpus callosum size. Men’s brains are 10-15% larger than women’s brain. In one recent study, neuroscientists compared the brains of 42 men and 58 women postmortem and found that men weighed an average of (3 lb), compared with (2.75 lb) for women Sundermann et al. [19].
On the minute structure, male brains are observed to have a slightly higher proportion of white matter, whereas females’ brains have a higher proportion of grey matter in most parts of the cerebral cortex. So, the cortex is slightly thicker in women's brains than in men's and is slightly more convoluted as well. There are also sex differences in the size of the individual brain structures. The hippocampus, a part of the brain that is involved in memory functions, is on the average, larger in men than in women, as is the amygdala, which is also involved in memory and emotions Guo et al. [20].
Another sex dimorphic brain structure is the third interstitial nucleus of the anterior hypothalamus. The function of this tiny part of the brain is unknown, but research from four different laboratories has repeatedly reported that it is almost twice as large in males as in females. It has also been linked to sexual orientation and gender identity Rawat et al. [21]. Another study found that it is smaller in MtF transsexuals, and larger in FtM transsexuals. These studies have been criticized for their small sample sizes, and the findings need to be confirmed by more studies Breedlove [22].
On a functional level, the observed brain sex differences will subsequently reflect differential cognitive abilities between women and men. For example, the language and visuo-spatial processing functions. Women are more empathetic and perform better on verbal memory and language tasks. While men are more aggressive and outperform women on mental tasks involving spatial skills such as mental rotation. Also, the corpus callosum is larger in women than in men. This was reported to go with that women are better at multitasking [23].
Also, studies showed that women are better than men at paying attention to sounds presented to both ears simultaneously Rigo et al. [23]. Investigations on the gray and white matters microstructure showed further sex-specific differences when assessing the gray matter volume, cortical thickness, diffusivity metrics of major fiber tracts, volumes of subcortical cell groups as well as neurochemical differences such as the serotonin transporter lateralization. These structural findings are complemented by the functional differences in the neuronal activation and the network characteristics of the functional and the structural connectivity Angelopoulou et al. [24]. The understanding of sex differences in the human brain makes us to understand the gender differences and the endocrine effects in the prevalence and the treatment of various psychiatric disorders. In this context, it is particularly interesting to study the Gender Identity Disorder (GID). It is a disorder characterized by a strong desire to belong to the opposite sex, which is often accompanied by emotional and social burden. So, these patients often seek hormonal treatment and sex reassignment surgery to feel more congruence between their gender identity and their appearance Schneider et al. [25].
Anatomical brain differences between the male and the female in brain imaging
The volume of the central subdivision of the Bed Nucleus of the Stria Terminalis (BSTc), a brain area that is essential for sexual behaviour, is larger in men than in women Garcia et al. [26]. A female-sized BSTc was found in a MtF (Male-to-Female) transsexuals. The size of the BSTc was not affected by the sex hormones in the adulthood and was independent of the at-birth sexual orientation. This denotes that a female brain structure in genetically male individual can lead to MtF transsexualism, and it also supports the hypothesis that gender identity develops as a result of an interaction between the developing brain and the sex hormones Uribe et al. [27]. Presence or lack of testosterone during the first 6–12 weeks of pregnancy, leads to the formation of a male or a female sex organs, respectively. In contrast, sexual differentiation of the brain occurs in the second half of pregnancy by the organizing effects of sexual hormones. Hence, these developmental processes are independent and chronologically separated, so that masculinization of the genitals may not necessarily reflect that of the brain Roselli [28].
Differences in the brain’s white matter that conflict with a person’s genetic sex may be a tool to identify the transsexual people before puberty Angelopoulou et al. [24].
Physicians are trying to find investigations to help children who feel that they are trapped in the body of the wrong sex. One key brain region involved in this respect is the BSTc, an area of grey matter, but the region is too small to scan in a living person, so differences have only been picked up at the postmortem investigations (Roselli [28]. Kreukels and Guillamon reviewed the neuro-imaging findings in people with gender dysphoria. They reported that the neuro-imaging studies of the brain structures revealed that the brain phenotypes of trans-women (MtF) and trans-men (FtM) differ in various ways from control men and women with feminine, masculine, demasculinized and defeminized features. Also, functional imaging studies may show whether brain activation and tasks performance in transgender people is sex atypical. They concluded that the brain phenotypes of people with feelings of gender incongruence may help us to figure out whether sex differentiation of the brain is atypical in these individuals [29]. Uribe and colleagues in February 2020 investigated the functional networks connectivity in the brain in a number of transwomen and transmen who have early-in-life onset gender incongruence. The investigators obtained MRI images from 29 transmen and 17 transwomen as well as 22 cisgender women and 19 cisgender men.
In discussion they concluded that transmen, transwomen, and cisgender women had decreased connectivity compared with cisgender men in superior parietal regions, as part of the Salience (SN) and the Executive Control Networks (ECN). Transmen also had weaker connectivity compared with cisgender men between intra-SN regions and weaker inter-network connectivity between regions of the SN, the Default Mode Network (DMN), the ECN and the sensorimotor network. Trans women had lower small-worldness, modularity and clustering coefficient than the cisgender men. There were no differences among Trans men, trans women, and ciswomen. Together these results underline the importance of the SN interacting with DMN, ECN, and sensorimotor networks in transmen, involving regions of the entire brain with a frontal predominance. Reduced global connectivity graph-theoretical measures were a characteristic of Tran’s women. It is proposed that the interaction between networks is a keystone in building a gendered self. This suggests that both proposed hypotheses are complementary in explaining brain differences between gender variants Uribe et al. [27].
In another study, the white matter of the brain was compared in 18 MtF transsexual persons with that in 19 males and 19 females. Surprisingly, in each transsexual person’s brain the structure of the white matter in the four regions was halfway between that of males and of females. “Their brains are not completely masculinised and not completely feminized, but they still feel female,” the investigators concluded Smith et al. et al. [30]. The Figure 1 and Table 1 show the structural connectivity networks in men and women. The figure shows the brain connectivity networks in transgender people. They show that males have higher within hemispheres and across-lobe connections, while in females; there is higher across-hemispheric lobar connections, according to the values of the LCW (Lobar Connectivity Weight) (ngalhalikar et al. and Hahn et al. (Figures 1 and 2) (Table 2) [31,32].
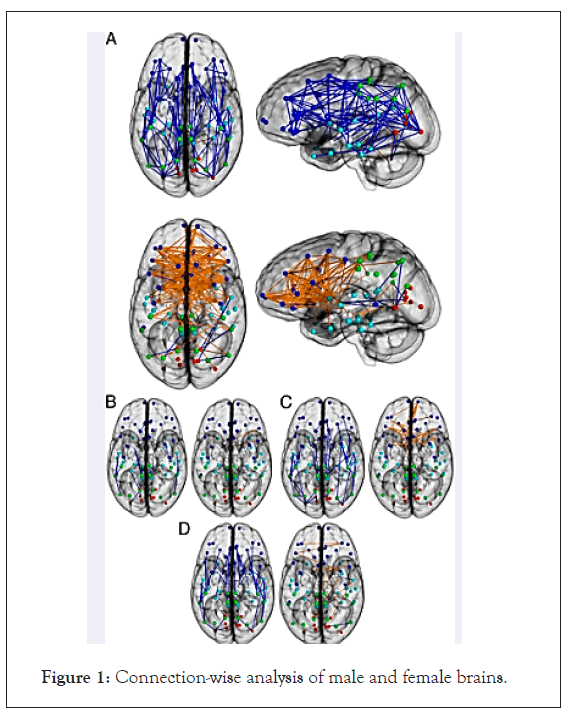
Figure 1: Connection-wise analysis of male and female brains.
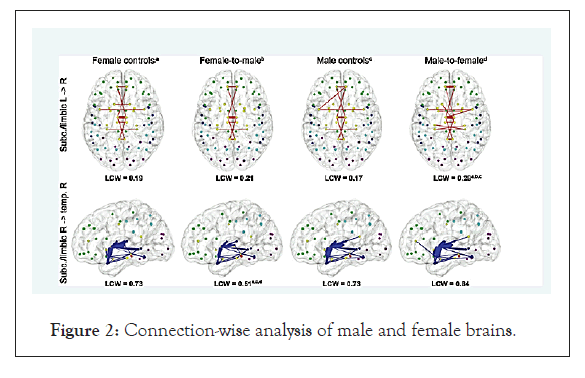
Figure 2: Connection-wise analysis of male and female brains.
Connection-wise analysis: (A) Brain networks show increased connectivity in males (Upper) and females (Lower). Analysis on the child (B), adolescent (C), and young adult (D) groups is shown. Intrahemispheric connections are shown in blue, and interhemispheric connections are shown in orange. Color representations are as follows: light blue, frontal; cyan, temporal; green, parietal; red, occipital; white, subcortical. GM, gray matter. Average structural connectivity for female controls, female-to-male transsexuals, male controls, and male-to-female transsexuals for full (i.e., unthresholded connectivity matrices). Increased LCW was found in maleto- female transsexuals a,b,c between right subcortical (subc.)/limbic and left subcortical/limbic lobes (red, top).
Decreased Lobar Connectivity Weight (LCW) was found in femaleto- male transsexuals a,c,d between right subcortical/limbic and right temporal lobes. Line thickness indicates connectivity weighting, whereas only connections with weights of >0.001 are shown (arbitrary choice to remove spurious connections as probabilistic tractography represents the robustness of the modeled tracts against noise). Nodes represent region of interest centers for frontal (green), temporal (blue), parietal (cyan), occipital (magenta), and limbic/subcortical brain regions (yellow). Characters cannot indicate differences as compared with female controls (a), femaleto- male transsexuals (b), male controls (c), or male-to-female transsexuals (d). Note that the axial images are in the radiological view (left image side is right hemisphere) (Figure 2) (Table 2).
This review demonstrated that the trans-sex is a true medical problem. Psychiatric and mental assessment will differentiate the mental disorders from the biological causes, an issue that should be known by all physicians. There are genetic, hormonal, and neurological causes that disturb the embryo development resulting into this problem. Trans-sex persons feel inconsistent with their assigned sex, and a desire for permanent transition to the opposite sex. It has social implications on person’s life, his family, job, and community. Trans-sex people face many challenges in their families, communities and health than the cis-gender counterparts. The need to change the society's look to the trans-sex people and the sex reassignment surgery are topics that will be discussed by the author in another review.
Campomelic dysplasia
A life-threatening disorder that affects development of the skeleton, reproductive system, and other parts of the body. This condition is often life-threatening in the newborn period.
Pleiotropy: The expression of multiple traits by a single gene.
The first author would like to acknowledge very much the second author; Professor Dr. Adel M. Elmansoury, for his broad mind, extended knowledge, and the sharp vision to suggest and give information and resources for this topic, that is not understood by many physicians till now.
There were no sources of funding neither during writing and preparation of this piece of work, nor during its editing, revising, submission for publication, nor any stage of its production.
There are no conflicts of interests regarding the topic of this work, nor its contents.
The first author collected the scientific sources, wrote, analyzed, reviewed, revised and submitted for publication, with follow up and replying to journals’ reviewers’ comments. The second author suggested the topic and highlighted its importance to be searched in and to spread the knowledge by it in the medical community. He read, revised, and approved what the first author wrote. He suggested the journal to be submitted.
Citation: Motawei SM, Elmansoury AE (2021) Trans-Sex: Time to Give Up Old Concepts. Andrology. 10:232.
Received: 30-Jul-2021 Accepted: 13-Sep-2021 Published: 20-Sep-2021 , DOI: 10.35248/2167-0250.21.10.232
Copyright: © 2021 Motawei SM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.