Organic Chemistry: Current Research
Open Access
ISSN: 2161-0401
ISSN: 2161-0401
Research Article - (2023)Volume 12, Issue 6
Threes newly azo derivatives were synthesized from this 1,2,4-triazole by converting the amine group to diazonium salt then reacted with various substituent phenol. The dyes have been characterized by IR, UV-Vis and 1H-NMR spectroscopic techniques as well as elemental analysis. In addition, the assessment of the antioxidant activity of the compounds (C1-3) by DPPH essay gave positive results comparing to ascorbic acid (VC) as a reference compound.
Triazole derivatives; Antioxidant; Azomethine; Ascorbic acid; Spectroscopy
The azo compounds are very important molecules and have attracted much attention in both academic and applied research [1-3]. These compounds are key chromophores in the chemical industry as dyes and pigments, food additives, indicators, radical reaction initiators and therapeutic agents, and have been used as cosmetics, in dying wool and wood, medicine industry as drugs to prevent the growth of germs [4]. The name of Schiff bases was given to the organic compounds containing the group of imine or (C=N) which is known also as the group of azomethine. The application of organometallic compounds in biology and medicine is nothing new indeed, nature has been using organometallic systems to sustain life for a rather long time. The organometallic chemistry of cobalamine, better known as vitamin B12, and derivatives has been investigated for decades, along with that of a variety of enzymes and cofactors containing metal-carbon bonds. Also the compounds which contain azo group and azomethine showed intensive interest in pharmacological and medicinal field besides they exhibited interested physical properties such as pH optical sensor, luminance and light emitting diodes and electro conductive properties [5,6]. According to the great importance of these compounds, this article presented synthesis of new 4- amino-1,2,4-triazole and their azo and azomethine derivative and investigated their thermal electro conductivity [7-9].
Materials and instrumentation: All reaction chemicals were used as received without further purification. Azo-coupled precursors (a1-3) were prepared according to the well-known procedure.
Instrumentation: IR spectra were measured by Bruker vertex 70. Elemental analysis was carried out using a euro vector E.A.3000 instrument using copper sample tubes. Melting points were measured by a Stuart scientific melting apparatus (uncorrected ± 0.1°C). The UV/Vis spectra were recorded in acetonitrile by using TIDAS fiber optic diode array spectrometer [10,11].
Synthesis of methyl 2-(2,3-dimethoxyphenoxy) acetate
Ethyl bromoacetate (16.7 g, 100 mmol) was added dropwise to a stirring solution of 2, 3-dimethoxy phenol (15.4 g, 100 mmol) and potassium carbonate (13.8 g, 100 mmol) in dry DMF 100 mL. The mixture was heated under refluxed at 70°C for 24 hs. The reaction was followed by TLC using hexane: Ethyl acetate (1:1) as eluent. After cooling the mixture was extracted by ethylacetate (2 × 50 mL). The combine organic layer was dried under magnesium sulfate. After removal of the solvent under reduced pressure affords pale yellow liquid. Yield (83%) (19.9 g); FT-IR (KBr, νmax): 3050, 3124 (CHAr), 2970, 2840 (CHAliph), 1751 (C=O), 1700, 1630 (C=C), 1236 (Ar-O-C), 1203 (C-O), 1119 (OCH3-OCH2) cm-1.
Synthesis of 2-(2 methoxy) acetohydrazide
Excess of hydrazine hydrate (80%) was added to a stirring solution of ethyl-2(2,3-dimethoxyphenoxy) acetate) (19 g, 79 mmol) in 40 mL of ethanol. The mixture was left under stirring overnight at ambient temperature. The solvent was reduced to half by evaporation under reduced pressure. The white solid precipitated was collected by filtration then washed with cold distilled water. The crud product was crystallized from methanol to afford white crystal yield (92%) (16.45 g): M.p. (104°C-106°C); FT-IR (KBr, νmax): 3427, 3325 (NH2), 3286 (NH), 3097, 3032 (CHAr), 2999, 2835 (CHAliph), 1655 (C=O) 1597, 1441 (C=C), 1304 (C-N), 1263 (Ar-O-C), 1117 (OCH3-OCH2) cm-1.
Synthesis of 2-hydroxy-3-((2,3-dimethoxyphenoxy)methyl)-1H-1,2,4-triazole-5(4H)thione
Excess of carbon disulfide (about, 3 ml) was added dropwise to a stirring solution of 2-(2,3-dimethoxyphenoxy) acetohydrazide (5 g, 22.1 mmol) and potassium hydroxide (22.1 mmol) in absolute ethanol (30 mL) at ambient temperature. The resulting precipitated was filtrated then washed by diethyl ether and dried. The crud potassium 2-(2-(2,3-dimethoxyphenoxy)acetyl) hydrazine carbodithioate salt (5 g, 14.7 mmol) was refluxed with 90 mL hydrazine hydrate 80% for 7 hrs until no more evolving of hydrogen sulfide gas which is tested by lead acetate paper. After cooling the product was poured into 100 mL crush ice. The pH of solution was adjusted to acidic medium (pH=4-5) using solution of 5% hydrochloric acid. The precipitate was filtrated, washed with cold water and dried under vacuum pressure. The precipitate was crystallized by methanol yield (70%), (2.9 g): M.p. (162°C-164°C) ; FT-IR (KBr, νmax): 3435, 3263 (NH2), 3215 (NH), 3066, 3026 (CHAr), 2968, 2837 (CHAliph), 2773, (C-SH), 1643 (C=N), 1601, 1496 (C=C), 1296 (C-N), 1259 (Ar-O-C), 1176 (C=S), 1103(OCH3-OCH2) cm-1.
Antioxidant activity
Biological harmonious chemistry is a wide field through its presence in most life systems, cancer preventing substances and tumors, metallic complexes with living systems that have an important role in the life of animals and plants. On the other hand, inorganic elements play a vital role in the fields of life and medical life, as it is clear that many organic compounds that are used in the field of medicine are not limited to organic mechanics only, but some organic compounds are active or biologically changed in the presence of metal ions, and it is indicated that some of the heavy transition elements in particular (the second and third chain elements) have a toxic effect in the body, so it is necessary to design coordinating compounds (coordinating agents) that have the ability to remove the toxic effect [12].
DPPH is a relatively free stable radical. It accepts hydrogen or an electron to be more stable. The DPPH percentage inhibition of the compounds (c1-3) and the standard compound (VC) are shown in the Table 1.
| Concentration (μM) | DPPH Scavenging effect (%) | |||
|---|---|---|---|---|
| 50 | C1 | C2 | C3 | Ascorbic acid |
| 150 | 22.7 | 34.9 | 17.9 | 40.6 |
| 250 | 19.6 | 32.6 | 16.7 | 43.6 |
| 350 | 26.7 | 37.1 | 15.5 | 46.9 |
| 450 | 29.9 | 40.9 | 19.2 | 49.4 |
Table 1: Scavenging activity of some synthetic compound.
The compounds (c2) proved a good antioxidant activity but the compounds c2 showed a better activity. However, the compound c3 displayed a less significant activity may be due to the substituent of Br on the phenyl ring.
Method of extraction
In cylindrical glass with a glass stopper, 5 ml of (10-4 mol/l) of the aqueous cation solution was added to 5 ml of the organic ligand solution, the mixture was shaken for 30 min at 25°C. The pH of the aqueous phase was measured and its metal concentration was determined by atomic absorption, and UV/visible spectrometry. This method was repeated three times, then the average value was calculated by equation:
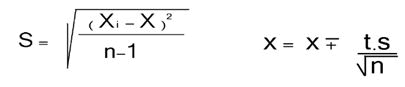
S: Average value.
t: Free degree.
Parameters of extraction
Distribution ratio (D): Distribution ratio refers to the ratio of cations between aqueous and organic phases, which is calculated by equation:
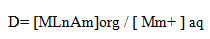
Extraction constant equilibrium constant for extraction will be given (Kex):
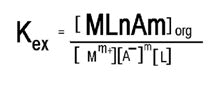
Where;
M+=Metal cation.
A-=Anions conjugated with cations.
L=Ligand.
N=The number of ligand moles.
[M]+=Cation concentration.
[Mm+][A-]m[L]n=Complex concentration in the organic phase.
Extraction percent (p%): The extraction percent for extraction will be given by equation:
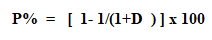
Preparation of extraction solutions
• Various concentrations of ligands (I), (II) and (III) were prepared in 1000 ml of toluene (0.5, 1, 1.5, 2, 2.5, 3) 10-4 mol/l by solving:
• (0.01564, 0.0378, 0.0422.0.0598, 0.07475, 0.0817) g, of ligand (I).
• (0.022, 0.045, 0.067, 0.081, 0.089, 0.099) g, of ligand (II).
• (0.027, 0.049, 0.057, 0.062, 0.088, 0.093) g, of ligand (II).
• Puffer solutions with pH (3-11) were prepared by adding (0.1) M of ammonia solution to 5 ml (0.1) M acetic acid, with 5 ml of (0.1) M NaCl, but HCl (0.1) M was used instead of acetic acid for solution with pH (1,2).
•(2 × 10-4) mol/l picric acid solution was prepared by solving 0.00458 g picric acid in 1000 ml of water (Figures 1-3).
Figure 1: Ligand concentration in chloroform (I).
Figure 2: Ligand concentration in toulene (II).
Figure 3: Ligand concentration in toluene (III)
We thought that it was prepared for the first time, and The prepared compounds were used to extract (Cu2+, Ni2+) cations. The best ratio (cation: ligand) was (2:1), and there was no increasing in the extraction percent above this ratio. The best aqueous pH was between (5-7) , and we could explain that from the ligand nature ,in acidic aqueous ,the nitrogen atom bond with protons, and in alkali aqueous the metals hydroxides were formed . Best time for copper and nickel was at half an hour, and any increasing in the mixing times didn't effect on the extraction percent. Extraction percents of copper were larger than of nickel in all cases.
In the present study, new azo-azomethine compounds were synthesized from the reaction of reducing the imine group. These compounds were characterized by IR, UV-Vis. The antioxidant activity of the compounds from (c1-3) was appraised and gave average results according to the following order c2>c1>c3.
The prepared compounds were used to extract (Cu2+, Ni2+) cations, the best ratio (cation: ligand) was (2:1), and there was no increasing in the extraction percent above this ratio. The best aqueous pH was between (5-7), and we could explain that from the ligand nature, in acidic aqueous, the nitrogen atom bond with protons, and in alkali aqueous the metals hydroxides were formed. Best time for copper and nickel was at half an hour and any increasing in the mixing times didn't affect on the extraction percent. Extraction percents of copper were larger than of nickel in all cases.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: AlAwad T (2023) Transition Metal Cations Extraction and Antioxidant Activity Studies of Some Triazole Derivatives. Organic Chem Curr Res. 12:338.
Received: 15-Sep-2023, Manuscript No. OCCR-23-23815; Editor assigned: 18-Sep-2023, Pre QC No. OCCR-23-23815; Reviewed: 04-Oct-2023, QC No. OCCR-23-23815; Revised: 12-Oct-2023, Manuscript No. OCCR-23-23815; , DOI: 10.35248/2161-0401.23.12.343
Copyright: © 2023 AlAwad T. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.