Journal of Infectious Diseases & Preventive Medicine
Open Access
ISSN: 2329-8731
ISSN: 2329-8731
Research Article - (2022)Volume 10, Issue 4
Introduction: Toxoplasmosis is an anthropozoonosis caused by Toxoplasma Gondi. Its transmission by cats and the existence of risk factors may be responsible for embryofetopathies of varying severity in Aboriginal women.
Objective: To analyze the risk factors and serology of toxoplasmosis in native pregnant women in two departments of the Republic of Congo.
Patients and methods: This was an analytical cross-sectional study conducted from January 1 to September 30, 2017 in 2 departments of the country, focusing on pregnant women divided into 2 groups 97 indigenous and 97 Bantu. The ELISA technique was used to study the sera of pregnant women. The variables studied were related to serology and risk factors. The statistical test was significant when p<0.05.
Results: TIZ decreased cellular ATP in a dose-dependent manner in MDCK cells and in MDCK cells infected with influenza A and B viruses. Maximum inhibition of ATP in influenza-infected or uninfected MDCK cells reached up to 45% after 6 and 24 hours of exposure to 100 μM TIZ. The decrease in cellular ATP did not affect cell viability and was reversible after eliminating TIZ from the culture. TIZ concentrations required to decrease cellular ATP levels were similar to those reported to inhibit replication of influenza A and B viruses.
Conclusion: The risk factors for toxoplasmosis are the same in native pregnant women as in Bantu pregnant women, despite the fact that the cat is more common in Bantu pregnant women.
Indigenous; Bantu; Pregnant risk factors; Serology; Toxoplasmosis
Toxoplasmosis is a cosmopolitan parasitic infection due to Toxoplasma Gondi that infects mammals and humans [1,2]. The risk factors for contamination are linked to certain eating habits, poor hygienic conditions, the presence of the definitive host (the cat) and the climate [1,3-5]. In pregnant women, the placental passage of the parasite leads to complications of varying severity, sometimes incompatible with life [1,2]. During pregnancy, diagnosis is based on the detection of specific Immunoglobulin’s (Ig) of type G and M [4-7].The seroprevalence of toxoplasmosis in pregnant women varies between 70%-80% and from one country to another in the general population [8-11]. Studies carried out in Quebec and Indonesia have reported a seroprevalence of 50% and 10.9% respectively among native pregnant women [12,13]. In the Central African sub-region, particularly in the Democratic Republic of Congo and Congo, it varies between 50 to 80% among the Bantu [2,3,5,14,15]. However, in the Congo, toxoplasmosis has not been studied in native pregnant women, yet their way of life could be an important determinant in its transmission in native pregnant women [16]. This is how we conducted the present study to analyze the risk factors and serology of toxoplasmosis in indigenous pregnant women in the Republic of Congo.
This was an analytical cross-sectional study, from January 1 to September 30, 2017. The study took place in the Republic of Congo in two departments: Lékoumou and Sangha. The samples were analyzed in the Parasitology Mycology department of the University Hospital Center of Brazzaville. The Department of Lékoumou is located in the south of the country with an area of 20950 km2. It is located 317 km from Brazzaville, accessible by land and air. It has 5 districts and 71,248 inhabitants, including 11,456 natives. The camps and neighborhoods of 3 districts of the department (Sibiti, Mayeye and Zanaga) were particularly the subject of the study. The Sangha Department is located in the north of the country, 643 km from Brazzaville with an area of 12,266 km2. It has 6 districts and 205,986 inhabitants, including 7,885 natives. The study concerned the urban community of Pokola located in the department of Sangha. This community has a population of 16,063 inhabitants, including 14,573 Bantu and 1,490 indigenous. Brazzaville is an autonomous department located in the south of the country. It is the political and administrative capital of the Republic of Congo. It has a University Hospital Center in which the Parasitology-Mycology laboratory is located, where toxoplasma serology was carried out.
Study population
The study population consisted of two groups: group 1 made up of indigenous pregnant women, and group 2, Bantu pregnant women. For each Aboriginal pregnant woman, we associated a Bantu pregnant woman. Were included all native and Bantu pregnant women, consenting to the study, whatever the age of pregnancy and who resided in the departments mentioned above. Indigenous and Bantu pregnant women whose sera were hemolyzed after centrifugation were excluded. For sample size calculation, we used the results of two studies conducted in the Democratic Republic of Congo (31% cat contact) [14] and Congo (60% seroprevalence) [15]. Using Schlesselman's formula [17] with P0=proportion of exposed in group 1=31%=0.3 and P1=proportion of exposed in group 2=60%=0.6 [15], the minimum sample size was N=47.
Study methods and materials
Pregnant women meeting the inclusion criteria were the subject of a double survey: epidemiological and biological. An on-site and home epidemiological survey was carried out using a standardized survey sheet. We conducted interviews in French (official language), Lingala, Kituba (national languages), and Bendjélé (indigenous dialect) with the help of an interpreter. A code and an identification number were assigned to each pregnant woman in order to guarantee data confidentiality and anonymity.
The indigenous and Bantu pregnant women selected benefited from blood samples of 5 ml at the bend of the elbow for the realization of toxoplasmosis serology in search of immunoglobulins G and M. We took blood samples in dry tubes from all indigenous and Bantu pregnant women in the departments of Lékoumou and Sangha. The tubes were centrifuged at 3000 rpm for five minutes. The sera were counted and stored at -20°C, transported to Brazzaville by plane in cooler containing cold packs. Arrived in Brazzaville, the sera were put in a freezer at -80°C until the day of the analysis. The sera were passed through an automated immuno-analysis (Mini- Vidas from Biomérieux®) to search for anti-toxoplasma antibodies (immunoglobulins G and M).
The automaton enabled detection and sandwich enzymatic immunoassay in two stages of antibodies of the Immunoglobulin G type (IgG) associated with a final detection in fluorescence (Enzyme Linked Fluorescent Assay: ELFA); and detection of serum Immunoglobulins M (IgM) by enzymatic immunoassay after immunocapture of the Ig M antibodies associated with final fluorescence detection (ELFA).
The IgM results were reported as an "i" index which indicates the ratio of the fluorescent signal of the tested serum to the stored signal of the standard. Any value i<0.55 corresponded to a serum free of antitoxoplasmic IgM; any value of i>0.65 corresponded to a positive serum in IgM. Any IgG value below 4 IU/ml corresponded to a serum free of antitoxoplasmic IgG; any value greater than 8 IU/ml corresponded to a serum positive for specific IgG. The intermediate immunoglobulin values corresponded to equivocal sera for which we did not perform a second assay three weeks later to know the patient's status. The results were considered “positive” when at least one class of Ig G or Ig M corresponded to the threshold for the presence of immunoglobulins using the Vidas Toxo technique from Biomérieux®.
Ethical consideration
The samples were taken in the Congo. There is no conflict of interest with the manufacturers of the equipment used and with the laboratories (Brazzaville). The tubes were destroyed immediately after analysis. Of the Research authorizations have been issued by the Faculty of Health Sciences, by the departmental health directorates of Lékoumou and Sangha.
Statistical analysis
Data entry was done from the software CSPRO. The software IBM SPSS version 20 was used for statistical analysis. Qualitative variables were expressed as frequency and quantitative variables as mean ± standard deviation. The significance level of the statistical tests was less than 5%.
We collected 200 sera from pregnant women, including 100 indigenous and 100 Bantu. 3 autochthonous pregnant sera were excluded because of hemolysis after centrifugation. We also removed 3 sera from Bantu pregnant women. We have therefore associated 1 autochthonous pregnant serum with a Bantu pregnant serum. The number of sera analyzed was 97 indigenous and 97 Bantu pregnant women. The median age was 27.7 ± 7.9 (14-41) among natives and 27.2 ± 6.7 (15-40) among Bantu. The most represented age groups were those of (20-25 years) and (26-31 years) (Figure 1). The risk factors related to the lifestyle of Aboriginal and Bantu pregnant women are given in Table 1. The overall seroprevalence was 54%, of which 23.1% among native pregnant women and 30.9% among Bantu pregnant women. The difference was not statistically significant (p=0.07). Lifestyle risk factors and seroprevalence of toxoplasmosis in Aboriginal and Bantu pregnant women, the estimated risk of toxoplasmosis in indigenous and Bantu pregnant women is shown in Table 2. Toxoplasmosis was observed more in Bantu than native pregnant women (Table 3).
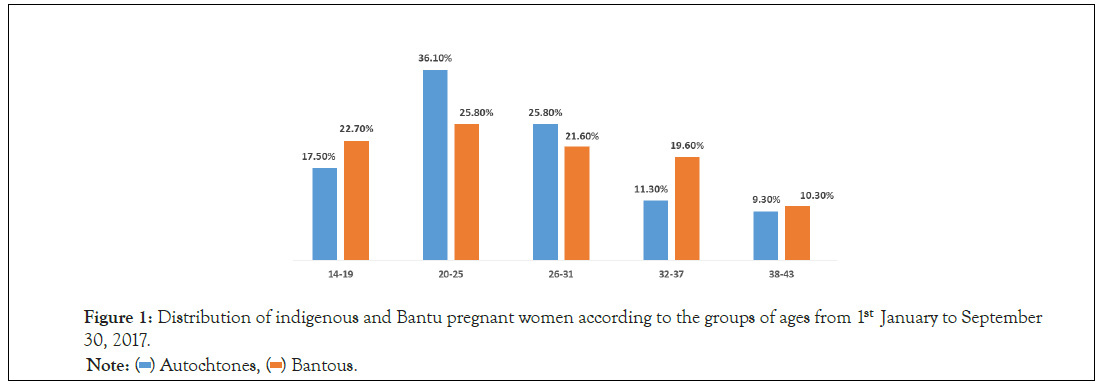
Figure 1: Distribution of indigenous and Bantu pregnant women according to the groups of ages from 1stJanuary to September 30, 2017.
| Educational level | Indigenous | Bantu | p | ||
|---|---|---|---|---|---|
| n=97 | % | n=97 | % | ||
| Illiterate | 48 | 49.5 | 9 | 9.3 | 0.001 |
| Primary | 49 | 50.4 | 44 | 45.4 | |
| Secondary | - | - | 25 | 25.8 | |
| Superior | - | - | 19 | 19.6 | |
| Religion | - | - | - | - | 0.001 |
| Animist | 36 | 36.4 | 5 | 5.2 | |
| Christianity | 63 | 63.6 | 92 | 94.8 | |
| Access to the health center | 67 | 69.1 | 97 | 100 | 0.001 |
| Gestational Age (Trimester) | - | - | - | - | 0.29 |
| First | 18 | 18.6 | 12 | 12.4 | |
| Second | 43 | 44.3 | 53 | 54.6 | |
| Third | 36 | 37.1 | 32 | 33 | |
| Prenatal consultation | 23 | 23.7 | 89 | 91.8 | 0.001 |
| Health worker qualifier | - | - | - | - | 0.001 |
| Doctor | - | - | 1 | 1 | |
| Midwife | 21 | 21.6 | 81 | 83.5 | |
| Nurse | 2 | 2.1 | 7 | 7.2 | |
| Toxoplasmosis serology not performed | 97 | 100 | 97 | 100 | <0.001 |
Table 1: Socio-sanitary and reproductive characteristics of indigenous and Bantu pregnant women from January 1 to September 30, 2017.
| Factors | Indigenous | Bantu | p | GOLD | CI | ||
|---|---|---|---|---|---|---|---|
| n=97 | % | n=97 | % | ||||
| Cat presence | 7 | 15.6 | 14 | 23.3 | 0.47 | 0.98 | 0.48-1.99 |
| Ground contact | 36 | 80 | 39 | 65 | 0.07 | 0.72 | 0.33-6.17 |
| Meat consumption | 4 | 8.9 | 1 | 1.7 | 0.3 | 1.43 | 0.37-1.39 |
| Cleaning hands after gardening | 29 | 78.4 | 40 | 100 | 0.001 | 0.78 | 0.66-0.92 |
| Hand washing after handling meat | 44 | 97.8 | 60 | 100 | 0.25 | 0.97 | 0.93-1.02 |
| Consumption of raw vegetables | 18 | 40 | 3 | 5 | 0.001 | 0.53 | 0.58-1.70 |
Table 2: Lifestyle risk factors according to the seroprevalence of pregnant women indigenous and Bantu from January 1st to September 30, 2017.
| Factors | Positive | Negative | p | GOLD | CI | ||
|---|---|---|---|---|---|---|---|
| n=105 | % | n=89 | % | ||||
| Sangha | 62 | - | 33 | - | 0.31 | 2.4 | 1.37-4.37 |
| aboriginal | 29 | 46.8 | 19 | 57.5 | - | 0.6 | 0.27-1.51 |
| Bantu | 33 | 53.2 | 14 | 42.2 | - | 1.5 | 0.65-3.62 |
| Lekoumou | 43 | - | 56 | - | 0.03 | 0.4 | 0.22-0.72 |
| aboriginal | 16 | 37.2 | 33 | 58.9 | - | 0.4 | 0.18-0.93 |
| Bantu | 27 | 62.8 | 23 | 41 | - | 2.5 | 1.07-5.46 |
| Together | 105 | - | 89 | - | 0.04 | 1.8 | - |
| aboriginal | 45 | 42.9 | 52 | 58.4 | - | 0.5 | 0.30-0.94 |
| Bantu | 60 | 57.1 | 37 | 41.6 | - | 1.8 | 3.33-1.06 |
Table 3: Risk of toxoplasmosis according to the departments according to seroprevalence.
The seroprevalence of toxoplasmosis was not statistically different in the two groups, although it was higher in the Bantu. Toxoplasmosis is not a rare pathology among natives. Literature data on this parasitosis are poor in this population because it is a population that lives in areas that are not always easy to access, but in addition, these peoples are protected in the majority of countries [18].
One of the causes of the rise in the seroprevalence of toxoplasmosis may be the low attendance of health structures by native pregnant women on the one hand, but also the absence of appropriate medical care. The presence of a single doctor in the health facilities where the study took place is an element that may be a risk factor for the occurrence of toxoplasmosis. The absence of qualified personnel therefore does not allow correct prevention information to pregnant women in the event of HIV-negativity. But also a lack of appropriate care in the event of seropositivity of toxoplasmosis serology. Contrary to what has been observed among the natives of Canada and Bali [13,19], Another remarkable element is that none of the pregnant women, whether indigenous or Bantu, from these localities had performed toxoplasmosis serology. The absence of screening for toxoplasmosis is a risk because most often toxoplasmosis is a pathology whose clinical expression is non- specific or asymptomatic. Toxoplasmosis can be identified early through serological examination [5,6].
The seroprevalence of toxoplasmosis was high despite the fact that the presence of the cat was not identified as a risk factor for toxoplasmosis in native pregnant women. This may be related to the native way of life where the cat is not the pet of choice. However, the cat has been identified as a major risk factor for toxoplasmosis, of which it is moreover the main host, as raised by a study carried out in Kinshasa [14]. This fact may suggest that in this population living mainly in forest areas, other felids could be the source of contamination. On the other hand, among native Indonesians, the cat has been found to be a risk factor for toxoplasmosis [13]. The only risk factors identified in our study are the consumption of raw vegetables and cleaning hands after gardening.
It should be noted that hand cleaning as a risk factor for toxoplasmosis raises the question of the quality of hand cleaning. Indeed, the oocysts of Toxoplasma Gondi being present in nature, they can be deposited on the hands. Poor cleaning of these would explain contamination, especially since the indigenous people are people who live by hunting, gathering and gardening [18]. The consumption of raw vegetables has been identified as a risk factor. Toxoplasma Gondi cysts are easily blown away by the wind, which can deposit them on vegetables, thus promoting consumption [6,14]. The irrigation of gardens with water contaminated by cysts has been a factor identified by some authors [4,5]. The observation that we have made is therefore not contrary to the data in the literature which raise the parasitic risks linked to food and lifestyle [2,6,13,19]. However, the natives do not practice large-scale agriculture which requires large irrigation networks. The factor therefore explaining the place of consumption of raw vegetables remains the dissemination by the wind.
Our study shows that toxoplasmosis is a reality in native pregnant women. The identified lifestyle risk factors for toxoplasmosis are the consumption of raw vegetables and the poor quality of hand cleaning after gardening. The seroprevalence of toxoplasmosis in indigenous pregnant women is low compared to Bantu. The serological status of native pregnant women was dominated by the absence of immunization. However, the presence of active toxoplasmosis is not negligible. Further studies would make it possible to search for the host responsible for the transmission of toxoplasmosis in natives and to study the status of newborns resulting from toxoplasmosis in pregnancy.
Citation: Obili SG, Rolland OIB, Potokoue-Mpia NSB, Nguesso I, Itoua C, loki LHI (2022) Toxoplasmosis and Pregnancy: Risk Factors and Serology among Indigenous People in Two Departments of the Republic of Congo. J Infect Dis Preve Med. 10:269.
Received: 17-Jun-2022, Manuscript No. JADPR-22- 17975; Editor assigned: 21-Jun-2022, Pre QC No. JADPR-22- 17975 (PQ); Reviewed: 06-Jul-2022, QC No. JADPR-22- 17975; Revised: 12-Jul-2022, Manuscript No. JADPR-22- 17975 (R); Published: 19-Jul-2022 , DOI: 10.35841/2329-8731.22.10.269
Copyright: © Obili SG, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.