
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Research Article - (2023)Volume 13, Issue 6
Background: AstroRx® is an allogeneic cell therapy, composed of healthy and functional human astrocytes derived from pluripotent embryonic stem cells. An intrathecal injection of a fresh formulation of AstroRx® cells for the treatment of Amyotrophic Lateral Sclerosis (ALS) was evaluated in an early-phase I/IIa clinical trial. The results of this study indicated that the treatment is safe and showed a signal of a clinical benefit in attenuating ALS progression. Due to the logistical challenges associated with the manufacturing and distribution of a fresh cell product and to allow completion of safety and quality control testing before cell administration, a cryopreserved formulation of AstroRx® was developed. The cryopreseved AstroRx® product includes 3.5% DMSO as a cryoprotectant. Upon thawing at the clinical site, the cryopreserved product is diluted before its use to achieve a concentration of 0.23% DMSO.
Objective: To evaluate the toxicity of DMSO-containing cryopreserved AstroRx® as compared to the fresh AstroRx® following their intrathecal injection into mice.
Methods: In vitro compatibility assessment between cryopreserved and fresh AstroRx® formulations, including cell viability, cell number, cell identity, impurities, safety and potency, was performed. In addition, a neurotoxicity assessment of intrathecal injection of DMSO alone was tested in immunocompetent Institute of Cancer Research (ICR) mice using two concentrations of DMSO, 0.25% and 0.5%. The neurotoxicity of DMSO-containing cryopreserved AstroRx® product was evaluated in immunodeficient NSG mice.
Results: In vitro comparability results demonstrated similarity between fresh AstroRx® (n=13) and cryopreserved AsrtroRx® (n=11) cell batches in all tested parameters. Intrathecal injection of DMSO at a concentration of 0.25% or 0.5% showed no difference, as compared to the control group, in food consumption, body weight, clinical symptoms, as well as neurological locomotor and beam tests, for 7 days post injection. Similarly, a single intrathecal injection of AstroRx® cryopreserved with DMSO following thawing or fresh AstroRx® to NOD scid gamma mice (NSG) mice was not associated with neurological signs or major systemic adverse effects during the 4 week study period. The presence of both fresh and cryopreserved AstroRx® cells at 4 weeks post injection was confirmed by Alu in-situ hybridization.
Conclusion: According to the study findings, intrathecal injection of a DMSO-containing formulation of cryopreserved AstroRx® cells does not appear to have a toxic effect on mice.
Astrocytes; Cryopreservation; Intrathecal injection; hESC; DMSO; Cerebrospinal Fluid (CSF)
ALS: Amyotrophic Lateral Sclerosis; APC: Astrocyte Progenitor Cell; CM: Cisterna Magna; CNS: Central Nervous System; CPA: Cryoprotective Agent; CSF: Cerebrospinal Fluid; DSMO: Dimethyl Sulfoxide; GFAP: Glial Fibrillary Acidic Protein; GLP: Good Laboratory Practice; GMP: Good Manufacturing Practice; hESC: Human Embryonic Stem Cells; ISH: In-Situ Hybridization; IT: Intra-Thecal; LD50: Lethal Dose of 50%; LP: Lumbar Puncture; MN: Motor Neuron; NSG: NOD Scid Gamma; PK: Pharmacokinetic
Intrathecal injection of human astrocytes (AstroRx®) derived from human embryonic stem cells to Amyotrophic Lateral Sclerosis (ALS) patients demonstrated a high safety profile, with a signal of beneficial clinical effect observed for the first 3 months following cell injection [1]. AstroRx® cells used for the trial were harvested, freshly formulated, and injected intrathecally to ALS patients within 24 hr from formulation. Disadvantage of using the fresh formulation, is the inability to perform long term sterility testing which are more accurate than shorter available testing, as well as synchronizing cell delivery and its timely administration. To permit coordination of cell administration with patient care and completion of safety and quality control testing before cell administration, the development of a cryopreserved off the shelf cell therapy is crucial for a successful delivery of cell-based therapies [2-4].
Cells that are cryopreserved are usually suspended in a defined solution containing agents that help the cells survive the stresses of the freezing and thawing processes (e.g., Cryoprotective Agents, CPAs). Numerous CPAs (e.g., Dimethyl Sulfoxide, Ethylen Glycol and Glycerol) as well as cryopreservation methods (e.g., controlled freezing or vitrification) have been under development with DMSO serving as a prominent one [5-8]. Dimethyl Sulfoxide (Me2SO, a.k.a DMSO) has dominated the biobanking of a variety of therapeutic cells, including CAR-T cells [9, 10], stem cells and stem cells derived cell products [11-14]. While in recent years several DMSO-free cryopreservants are available, their formulation is also based on the presence of a cryopreservant, which is mostly not as well characterized [15]. Human Serum Albumin (HSA) is also a common ingredient in cryopreservation media, used as a regulatory-approved replacement for Fetal Bovine Serum (FBS) [16,17].
DMSO is a compound of low toxicity registered in the ICHQ3C guideline in the category of solvents of low toxicity with no known molecular targets. The median Lethal Dose (LD50) values following Intravenous (IV) dosing in animals are in the range of 1-5.6 gr/Kg [18]. Yet, it is not an inert molecule and has pleiotropic effects in many biological systems [19,20]. Limited information is available about the use of DMSO for intrathecal injection. DMSO is commonly administered intravenously at high concentrations to humans, up to 1 gram per kg body weight per day; with the rate of infusion not exceeding 5 mL/kg/hr, most reported adverse events are mild [21]. In addition, clinical experience is available for intravesical DMSO administration such as use of RIMSO-50, DMSO 50%, an FDA approved treatment for interstitial cystitis. However, in this case, the distribution of DMSO to other tissues is very limited [22]. In addition, there is a hematopoietic progenitor cell Injectable cell suspension (DUCORD, Cord Blood), suspended in 10% DMSO. The DUCORD package notice states that the maximum tolerated dose of DMSO has not been established, but it is customary not to exceed 1 gr/kg/day when given intravenously.
Pharmacokinetic (PK) analysis of IV bolus of 1.5 gr/kg DMSO in mice showed that DMSO was rapidly and extensively distributed to mouse tissues, including brain with Maximum Tolerated Dose (MTD) of 1 gr/kg/day DMSO given IV being considered safe [23]. Administration of DMSO by the intrathecal route is less common and most cryopreserved cell therapy administered by this route includes a washing step to remove the DMSO [24,25].
Here we report on the development of a cryopreserved off-theshelf cell therapy (AstroRx®) which is based on DMSO as the cryoprotective agent. To mitigate potential risk of intrathecal injection of DMSO as an excipient in the intended clinical dose, we tested the neurotoxicity effect of DMSO alone in immunocompetent ICR mice. Then, the toxicity of AstroRx® cryopreserved with DMSO at the intended clinical use was tested in immunocompromised NSGTM mice for up to 4 weeks following intrathecal injection.
Manufacturing of AstroRx® (Fresh and cryopreserved)
Derivation of AstroRx® was performed according to previous published protocol [1,26]. In brief, HADC-100, a clinical grade line of human embryonic stem cells (obtained from the Hadassah Medical Organization, Jerusalem) was expanded in a feeder free condition with Essential 8™ (E8) medium (Thermo Fischer Scientific). Once sufficient number of hESC cells was obtained, Astrocyte Progenitor Cell (APC) banks were generated. This was done by first transferring hESC colonies into 100 mm ultralow attachment culture plates (Corning) containing ITTSPP/B27 medium. ITTSPP/B27 is a mixture of DMEM/F12 containing 1% B27 supplement, 1% Glutamax, 1.5% Hepes at pH 7.4 (all from Thermo Scientific), 1% penicillin/streptomycin/amphotericin solution (biological industries), 25 μg/ml human insulin (ActRapid; Novo Nordisk), 50 μg/ml human apo-transferrin (Athens), 6.3 ng/ ml progesterone, 10 μg/ml putrescine, 50 ng/ml sodium selenite and 40 ng/ml triiodothyronine (T3) (all from Sigma). ITTSPP/ B27 was supplemented with 20 ng/ml r-human EGF (R&D systems). After 2 days, the medium was changed to ITTSPP/B27 supplemented with 20 ng/ml EGF and 10 μM ATRA (sigma). The culture was continued in suspension in the nonadherent plates for 7 days with daily replacement of the medium. During the last step, which allows for Neurospheres (NS) ripening, the culture was continued in ITTSPP/B27 medium supplemented with 20 ng/ml EGF for 18 days with media replacement every other day. Then round yellow NS were manually selected using a stereoscopic microscope and transferred into laminin 521 (biolamina) coated flasks cultured in ITTSPP/B27 supplemented with 20 ng/ml EGF. Medium was replaced every other day for 7-10 days (passage 0). In order to produce a monolayer, the spheres were dissociated with TryplE (thermo scientific) and reseeded on laminin 521 coated plates in N2/B27 medium consisting of DMEM/F12 with 0.5% (v/v) N2 supplement, 1% (v/v) B27 supplement, 1% Glutamax and 1.5% Hepes at pH 7.4 (all from Thermo Scientific). The growth factors EGF and bFGF (R&D Systems) were added at 10 ng/ml each. The monolayers of APCs were further passaged weekly until a sufficient number of cells were obtained to freeze the APC cell banks.
AstroRx® generation involves the differentiation of APCs into committed astrocytes. APC cell bank vial was thawed, seeded and expanded on flasks coated with human recombinant laminin-521 (Biolamina) in N2/B27 supplemented with Recombinant human EGF and FGF both at concentration of 20 ng/ml as well as 2% irradiated human male AB Off-The-Clot (OTC) Serum (Access Cell Culture, AC-002-1B-GI). To promote astrocyte differentiation, the Epidermal Growth Factor (EGF), basic Fibroblast Growth Factor (bFGF), and irradiated human AB serum are removed from the media and sodium ascorbate (Vitamin C) is added. Cells are then grown for 7 days without growth factors. For the fresh AstroRx® cell product, the cells are harvested and formulated in PlasmaLyte to reach a concentration of 40 × 106/ml. For the cryopreserved off the shelf product, AstroRx® is cryopreserved in Plasma-Lyte supplemented with 3.5% DMSO and 9.3% Human Serum Albumin (HSA). To minimize manipulations steps at the clinical site thawed cells are diluted in PlasmaLyte (1:15) prior to injection, accordingly, the final AstroRx® cell therapy contains 0.23% DMSO and 0.62% HSA in 40 × 106/ml cell concentration. PlasmaLyte only or PlasmaLyte with 0.23% DMSO and 0.62% HSA were used for intrathecal injection in NSG mice. For neurotoxicity in immunocompetent mice, 0.25% or 0.5% DMSO in PlasmaLyte were used.
Characterization of AstroRx® and vehicle controls
Validated safety quality control tests were performed before the release of each formulated AstroRx® cell product and vehicle controls before injection, including sterility and endotoxin, performed by external qualified and certified GLP laboratory (Hylabs laboratories, Israel). The viability and cell concentration were determined using an automated cell counter Nucleocounter (NC-200™ Chemometec®). The identity of AstroRx® cells was assessed by flow cytometry using the following antibodies: Anti- GLAST (Miltenibiotec, 1:100), anti-CD44 (BD Pharmingen, 1:50), and anti-GFAP (Miltenibiotec, 1:50). Antibodies against SSEA-4 and EPCAM (both from Biolegend) were used for the detection of any pluripotent marker impurities. The Flow cytometer FACS Canto II (BD) operated with FACSDIVA software (BD) was used for the analysis. To assess AstroRx® potency in-vitro, AstroRx® cells secretion of Midkine and TIMP-1 was determined by ELISA using Human TIMP-1 Quantikine ELISA Kit (R&D systems) and Human Midkine ELISA Kit (Abcam). The optical density was read using the iMark Microplate reader (Bio-Rad Laboratories). A certificate of analysis was generated and approved by the quality assurance department to ensure that each released product met the release criteria before it was delivered for Intrathecal injection.
Neurotoxicity study of DMSO in immunocompetent mice
Intrathecal injection of vehicle controls: Animal handling was performed according to guidelines of the National Institute of Health (NIH) and the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC), the study was performed at Science In Action (SIA) facility (Weizmann Science Park in Ness Ziona, Israel) under the approval by "The Israel Board for Animal Experiments", in compliance with "The Israel Animal Welfare Act” and Ethics Committee (Approval no. IL-b18912-16), as well as in accordance with ARRIVE guidelines. Thirty eight (38) weeks old ICR mice were used for the study. Ten animals were randomly allocated to each experimental group. 7 µl of vehicle controls (PlasmaLyte, PlasmaLyte with 0.5% DMSO or PlasmaLyte with 0.25% DMSO) were injected into mice Cerebrospinal Fluid (CSF) through the Cisterna magna. At the end of this study animals were released and not euthanized.
Animal procedures: Mice were anesthetized with a mixture of 2% Isoflurane and 3% oxygen. Each mouse was placed in a stereotactic device and the head of the mouse was shaved and disinfected with 70% alcohol. An incision was made along the head of the mouse and the cisterna magna was exposed. The injection was performed with the Hamilton syringe, using a 26G needle with a volume of 7 µl/mouse.
Evaluation and behavior tests survival was recorded
Clinical evaluation: Parameters such as mice activity, nest building, interaction with cage mates, general appearance, fur appearance, body condition, observable anomalies were assessed on a daily base and served as indicators of general health and well-being. In addition food consumption and body weight were evaluated.
Locomotor test: The open field locomotor test activity comprehensively assesses locomotor and behavioural activity levels of mice. The test measures the distance the animal moved, movement pattern and the velocity. On the day of the test, each animal was transferred to an isolated and quiet behavior room and placed in an open space cage (35 cm × 25 cm) that is digitally divided into 14 sections. The cage was photographed for 10 minutes by a camera connected to Ethovision software. The software calculates the total duration of the movement of the animal for 10 minutes, and the number of times it transits from section to section.
Beam test: The test measures the time it takes (score in secs) for the mouse to reach from one side of the beam to the other. On the day of the test each animal was transferred to a secluded and quiet behavior room. The test was performed using a beam (50 cm long and 6 mm wide). On both sides of the beam there is a dark box with an entrance opening. The animal was placed at the beginning of one side of the beam, and a stopwatch was activated to measure the time the animal passes to the other side of the beam. The test was conducted 3 times for each animal tested sequentially, and the shortest time was recorded. Body weight, beam test and locomotor test were performed 5 days prior to treatment (-5) and 1, 2, 3 and 7 days post-treatment. Animals were released and not euthanized at the end of this study.
Statistical analysis: Data are presented in average and Standard Error of the Mean (SEM). T-test statistical analysis was performed between tested treatments and control treatments. Results with p value smaller than 0.5 were defined as having significant statistically difference.
Toxicity assessment of fresh, cryopreserved AstroRx® and their respective vehicle controls following intrathecal injection to NSG mice
This study was performed under GLP conditions at Envigo CRS Israel, and under the approval of the National Council for Animal Experimentation (No. IL-2112-132-4), in accordance with ARRIVE guidelines. Toxicity was tested on 4 experimental groups: Two test item (Fresh and Cryopreserved AstroRx®) and two vehicle control groups. All groups were subjected to a single Intrathecal (IT) injection into the CSF through the Cisterna Magna (CM). All animals were injected subcutaneously with an analgesic agent (~0.05-0.1 mg/kg of buprenorphine), at least 30 minutes prior to CM administration. Animals were anesthetized by isoflurane inhalation (2-3% in oxygen at a flow rate of 0.8-1.2 L/min.). Each material group comprises n=20 animals of 10 male and 10 female mice. Animals were observed for duration of up to 28 days. Animals were injected with 10 μl of the 0.4 × 106 cells/10 μl/animal of cryopreserved or fresh AstroRx® cells (eqvivalent to human cells dose of 500 × 106 cells) or vehicle controls. Additionally, an untreated control group (naïve mice) was added for species basal levels (n=10). All animals were subjected to assessment of systemic clinical signs, neurological examination, body weight, and determination of food consumption during the study period. Five male and 5 female mice from each treatment group were sacrificed one day following dosing. The remaining 5 male and 5 female mice in each group and the naïve mice were sacrificed four weeks later, to assess any adverse reactions or delayed toxicity effects. The Study design is given in Supplementary Table 1. At each respective time point of termination, blood for clinical pathology tests was collected and analyzed by American Medical Laboratories (AML, Israel). In this study none of the Humane Endpoints (HEP) were reached. Humane Endpoints included: Moribund condition, predictable or impending death, signs of severe pain, body weight, unexpected severe clinical signs (e.g., diarrhea, dyspnea, etc.) and severe neurological clinical signs (e.g., tremors, twitches, convulsion, etc.).
| AstroRx® cell characterization | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total cell number (106) | Viability (%) | Identity | Impurities | Potency | Safety | |||||||
| CD44 (%) | GLAST (%) | GFAP (%) | SSEA-4 (%) | EpCAM (%) | TIMP-1 (ng/1M cells) | Midkine (ng/1M cells) | Sterility | Endotoxin (LAL) EU/mL | Mycoplasma | |||
| Release criteria | 100 ± 20% | ≥ 80 | ≥ 85 | ≥ 70 | ≥ 75 | ≤ 0.1 | ≤ 0.1 | ≥ 5 | ≥ 0.5 | No growth | ≤ 0.5 | Not detected |
| Cryopreserved AstroRx® | 102 ± 3.7 | 88.2 ± 1.1 | 98.8 ± 0.2 | 90.5 ± 1.4 | 90.2 ± 1.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 83.3 ± 7.8 | 11.5.0 ± 1.5 | No growth | <0.5 | Not detected |
| Fresh AstroRx® | 98.2 ± 1.8 | 94.3 ± 0.8 | 99.3 ± 0.1 | 78.3 ± 4.7 | 94.7 ± 0.9 | 0.0 ± 0.0 | 0.0 ± 0.0 | 43.9 ± 4.8 | 17.2 ± 1.2 | No growth | <0.5 | Not detected |
Table 1: Comparison between fresh and cryopreserved AstroRx® Cells.
Carbon dioxide (CO2) inhalation is the most common method of euthanasia and was used at the end of the study (according to AVMA guidelines for the euthanasia). Animals were euthanized by trained personnel using appropriate technique and equipment. Upon completion of the procedure, death was confirmed by ascertaining respiratory arrest and noting an animal's fixed and dilated pupils. All the animals were subjected to necropsy and macroscopic pathological evaluation. Selected organs from all animals (excluding the naïve mice) were submitted for histopathological evaluation performed by Abraham Nyska, Toxicologic Pathology Ltd, Israel. Brain and spinal cord of selected animals assigned to the cryopreserved AstroRx, and respective vehicle control groups were also subjected to In-Situ Hybridization (ISH) analysis for Alu y gene for the detection of injected cells (StageBio, MD, USA).
Clinical signs examinations
Observations include changes in skin, fur, eyes, mucous membranes, occurrence of secretions and excretions (e.g., diarrhea), and autonomic activity (e.g., lacrimation, salivation, piloerection, unusual respiratory pattern). Changes in gait, posture, and response to handling, as well as the presence of peculiar behavior, tremors, convulsions, sleep and coma were also observed and recorded.
Neurological assessment
Neurological assessment was performed for all animals assigned to 28-days termination time point. The neurological examination was based on a Function Observation Battery (FOB) (The Irwin test and Functional Observational Battery (FOB) for assessing the effects of compounds on behavior, physiology, and safety pharmacology in rodents. Joanne R Mathiasen , Virginia C Moser). Observations were scored according to a semi-quantitative grading of five grades (0-4)-0: Normal or none, 1: Minimal or slight, 2: Moderate, 3: Marked, 4: Extreme.
Hematology and biochemistry analysis
Hematology and biochemistry was performed on parameters listed in Supplementary Table 2. Individual blood samples (at least 100 μl whole blood, collected into commercial EDTA-coated tubes for hematology and at least 500 μl whole blood, were collected into commercial serum separation gel tubes and centrifuged for separation of at least 200 μl serum for biochemistry) were obtained by retro-orbital sinus bleeding under isoflurane inhalation (1%-3% in oxygen), before termination. Each vial, of the final blood sample, was identified by Study No., Group No., Animal No. and Date of Necropsy. Following completion of blood collection, all samples (whole blood and serum samples) were analyzed by American Medical Laboratories Ltd (Israel).
| Group No. and sex | Group size | M O R T A L I T Y number affected/total number of animals |
|---|---|---|
| 1M (Intact) | n=5 | 0/5 |
| 2M | n=10 | 0/10 |
| 3M | n=10 | 0/10 |
| 4M | n=10 | 0/10 |
| 5M | n=10 | 0/10 |
| 1F | n=5 | 0/5 |
| 2F | n=10 | 0/10 |
| 3F | n=10 | 0/10 |
| 4F | n=10 | 0/10 |
| 5F | n=10 | 0/10 |
Note: M: Male; F: Female.
Table 2: Mortality Incidences.
Histological processing and evaluation
All organs and tissues (adrenals, brain, spinal cord, epididymitis, heart, kidneys, liver, lungs, ovaries/testes, salivary glands and spleen) were collected from all animals and fixed in either 10% neutral buffered formalin (approximately 4% formaldehyde solution) or Davidson’s Solution for at least a 48 hr fixation period prior to their shipment to histological processing at StageBio (MD, US). The tissues were trimmed, embedded in paraffin, sectioned at approximately 5 microns thickness, and stained with Hematoxylin and Eosin (H&E). Brain regions were sampled bilaterally using 7 coronal sections. The spinal cord was transversely sectioned at the cervical, thoracic, lumbar and sacral areas, at 3 sections per area. Microscopic histopathological changes were described and scored using a semi-quantitative grading of five grades (0-4): Grade 0 is no abnormalities and grade 1,2,3,4 is minimal, mild, moderate and severe respectively.
In-situ hybridization examination: In-Situ Hybridization (ISH) with human-specific Alu probe for detection of human cells was conducted to the organs: Brain and all parts of the spinal column (i.e., cervical, thoracic, lumbar, and sacral) from animals assigned to the 28-days termination time point of groups 3, 4, and 5 (n=2 per group per sex-total of 12 mice, first two mice in each group).
Statistical analysis: Statistical analysis was performed using Outlier. Rnw (validated R-Script for evaluating outlier data points, Version 1) T Test. Rnw (validated R-Script for statistical evaluation between 2 groups, Version 1) and MultiComp. Rnw (validated R-Script for statistical evaluation between multiple groups and/ or multiple parameters between 2 groups). Prior to application of the appropriate statistical method a normality test was performed considered Gaussian distribution (e.g., Shapiro-Wilk normality test; p<0.01).
Development of cryopreserved off the shelf cell product
To allow completion of all safety and quality control testing before cell administration as well as to provide higher flexibility in coordinating AstroRx® injections, a cryopreserved AstroRx® off-the-shelf cell therapy was developed. For the cryopreserved off-the-shelf product, AstroRx® was cryopreserved in Plasma- Lyte supplemented with 3.5% DMSO and 9.3% Human Serum Albumin (HSA). To minimize manipulations steps at the clinical site, we chose to simply dilute the thawed cells in PlasmaLyte (1:15).
Accordingly, the final AstroRx® cell therapy product contains 0.23% DMSO and 0.62% HSA at 5 × 106/ml cell concentration. The stability (in term of cell number and viability) was determined to be for 4 hours after thawing and dilution. Comparability results presented herein, support the similarity of fresh AstroRx® (n=13 batches) and cryopreserved AsrtroRx® cell therapy (n=11 batches) by demonstrating the conservation of product quality attributes (viability, cell number, cell identity and impurities) as well as safety and potency (Table 1). Cryopreserved AstroRx® cell therapy met the release criteria defined for AstroRx® cell product with a stability of 4 hours in room temperature after thawing and dilution (Table 1).
IT neurotoxicity of DMSO in immunocompetent mice
To evaluate the neurotoxicity of intrathecal injection, DMSO at two concentrations (0.25% similar to intended clinical dose and 0.5%) as well as Plasma-Lyte only (control) were injected intrathecal to immunocompetent mice. No significant differences in food consumption over 24 hours were determined between control and tested treatments on days-5, 1 and 7 (Figure 1A). On days when animals were weighed (days-5, 1, 2, 3 and 7), the animals were observed for general behavior, motility, hair, skin lesions, tremor, diarrhea and drooping eyelids. No external side effects/observations were recorded in all treated animals (not shown). No significant differences in body weight were determined between control and tested treatments on each of the tested days (Figure 1B). There was no significant difference in locomotor test, determined by the mean velocity or distance between study groups on any of the tested days (Figures 1C and 1D). A significant decrease in performance was observed when comparing naïve animals before to after intrathecal injection but this was found in all tested groups (including controls without DMSO). This result might be explained by the intrathecal injection procedure itself that limited mice mobility. No significant difference was observed in the beam test assay between shams injected and treated groups on days-5 (before treatment), day 1, day 2 and day 3. On day 7 a significant difference in the results was observed between sham control and treated mice (Figure 1E). This difference was apparently due to two out of the ten mice in the group that walked more slowly relative to the rest of the group and may be considered as outliers. Thus, overall, we concluded that the results between treatments and days were comparable indicating no meaningful difference between control and DMSO treated animals.
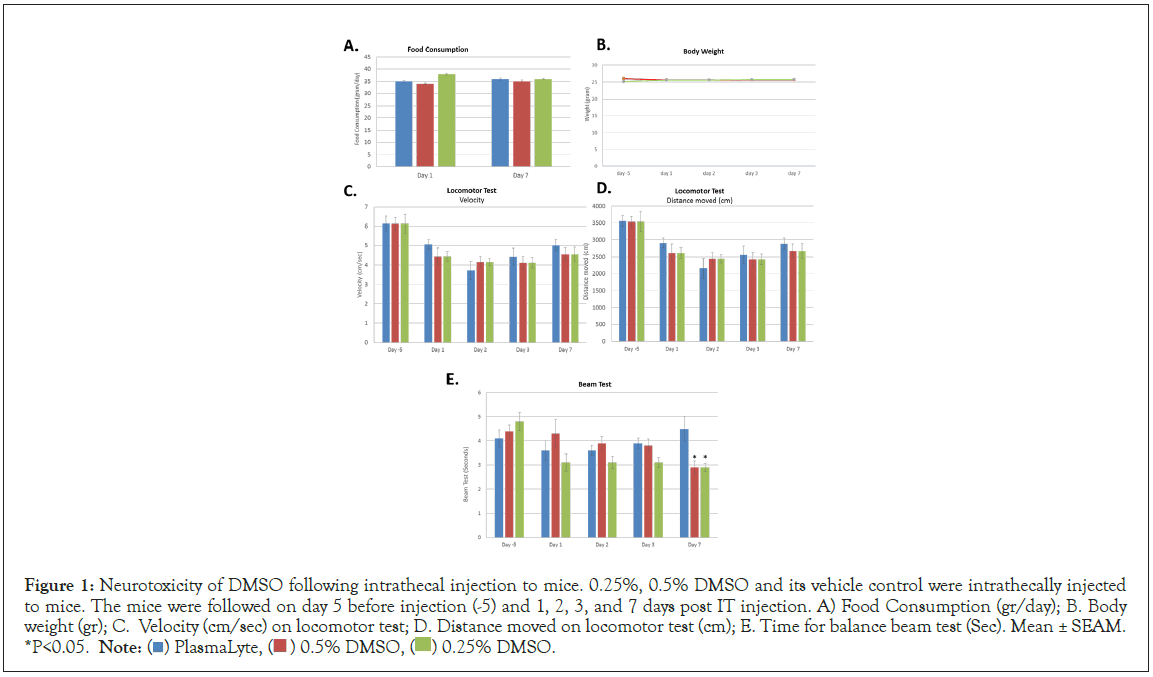
Figure 1: Neurotoxicity of DMSO following intrathecal injection to mice. 0.25%, 0.5% DMSO and its vehicle control were intrathecally injected
to mice. The mice were followed on day 5 before injection (-5) and 1, 2, 3, and 7 days post IT injection. A) Food Consumption (gr/day); B. Body
weight (gr); C. Velocity (cm/sec) on locomotor test; D. Distance moved on locomotor test (cm); E. Time for balance beam test (Sec). Mean ± SEAM.
*P<0.05. Note:  DMSO.
DMSO.
Toxicity of cryopreserved AstroRx® in NSG mice
The objective of this study was to assess the potential toxicity of cryopreserved AstroRx® cells, in comparison to fresh AstroRx® cells and its vehicle controls following a single Intrathecal (IT) injection into the Cerebrospinal Fluid (CSF) through the cisterna magna of male and female NSG (NOD SCID gamma) mice. In this GLP study, no mortality occurred throughout the entire observation period (Table 2). In most of the neurological parameters, no significant difference was found and was comparable between the groups. Few sporadic findings were observed in all tested groups in female’s mice in form of hypotonic gait and hind limb reflex moderate with no findings in the histopathological evaluation of the brain, spinal cord and sciatic nerve (Supplementary Table 3). No abnormal clinical signs were observed one-day post-dosing and throughout the 28-day observation period, excluding one female from group 5 (cryopreserved AstroRx®) which was observed not stepping on its right foot for 3 days, starting 4 days post-dosing until day 7 post-dosing, this event was temporary and considered incidental (Table 3). No statistically significant difference was noted in mean group body weight gain at the termination of the study between male and females from both AstroRx®-treated groups vs. the control groups (Supplementary Table 4), excluding a statistically significant increase in weight of females of group 4 (Fresh AstroRx) weight vs. vehicle controls (group 2 and group 3), this difference was considered incidental. Blood samples for hematology biochemistry tests were collected on 1-day post dosing and 28-days post-dosing. There were some statistically significant changes relative to the control groups in hematology and biochemistry parameters. These changes were minor, comparable to the naïve group and were not consistent between the two sexes, thus are not considered to be related to treatment. These changes include a statistically significant increase in triglyceride levels vs. the control groups that was noted in male mice from group 5M, treated with cryopreserved AstroRx®, 28-days post-dosing (Supplementary Tables 5-12). This elevation is mainly attributed to two male animals which displayed high triglyceride levels. Since this change was not accompanied with an increase in cholesterol or liver enzymes and was not observed in the females from the same group, it can be considered as an incidental finding. All groups exhibited an increase in WBC count 1-day post-dosing (both males and females) due to elevation in neutrophil count which may be stress induced due to anesthesia and intrathecal injection procedure (Supplementary Tables 5-12). No gross pathological findings were observed in any of the animals at the time of their scheduled necropsy (Supplementary Table 13).
| Group No. and sex | Observation | On study day number affected/total number of animals (days post dosing) | |||
|---|---|---|---|---|---|
| 0 | 1-3 | 4-6 | 7-27 | ||
| 1M | NAD | 5/5 | 5/5 | 5/5 | 5/5 |
| 2M | NAD | 10/10 | 5/5 | 5/5 | 5/5 |
| 3M | NAD | 10/10 | 5/5 | 5/5 | 5/5 |
| 4M | NAD | 10/10 | 5/5 | 5/5 | 5/5 |
| 5M | NAD | 10/10 | 5/5 | 5/5 | 5/5 |
| 1F | NAD | 5/5 | 5/5 | 5/5 | 5/5 |
| 2F | NAD | 10/10 | 5/5 | 5/5 | 5/5 |
| 3F | NAD | 10/10 | 5/5 | 5/5 | 5/5 |
| 4F | NAD | 10/10 | 5/5 | 5/5 | 5/5 |
| 5F | NAD | 10/10 | 5/5 | 4/5 | 5/5 |
| Did not stepped on right foot for 3 days | 0/10 | 0/5 | 1/5 (# 134) | 0/5 | |
Note: NAD: No Abnormality Detected.
Table 3: Clinical signs and cage side observation.
In the histopathologic findings, the intrathecal administration was associated with rare cases of minimal accumulations of meningeal histiocytes. These lesions, mostly confined to the 28-day sacrifice male animals, were usually focal, and were not associated with any damage to the adjacent brain tissue. As shown in the Figure 2, the cells were relatively large, with finely vacuolated and/or eosinophilic cytoplasm, consistent with structure of histiocytes (i.e., antiinflammatory type II (M2) phenotypes, M2-like-macrophages). The M2 macrophages are part of the response to absorbable implants, and often involves large or foamy macrophages (i.e., containing phagocytized products), and contribute to tissue repair and healing, and are not part of pro-inflammatory response [27,28]. Such changes were also seen in a female animal from group 2, treated with vehicle (PlasmaLyte only), and it is suggested that this change is related to reaction associated with the intrathecal administration procedure and not to any of the test compound components [29]. Due to the minimal grade, without damage to the adjacent tissue, this change is judged to be not adverse, according to the criterial of the Society of Toxicologic Pathology (STP) [30]. All other observed changes were considered as spontaneous, characteristically seen in control mice of this strain [31]. The presence of AstroRx® injected cells after 28 days was verified by ISH of Alu probes. Alu elements are primate-specific short, interspersed elements which are present in the human genome and serve as a useful target for detecting human cells [32]. Positive Alu labeled cells were detected in the brain, in both group 4 (Fresh AstroRx®) and group 5 (Cryopreserved AstroRx®) injected animals, indicating AstroRx® cell survival (Figure 3). These results are in line with previously reported data [26]. No Alu positive cells were identified in the spinal cord sections from any animal from all groups. The use of formical to decalcify the spinal cords in these animals may have had an impact in reducing the detectability of the ISH RNA target [33].
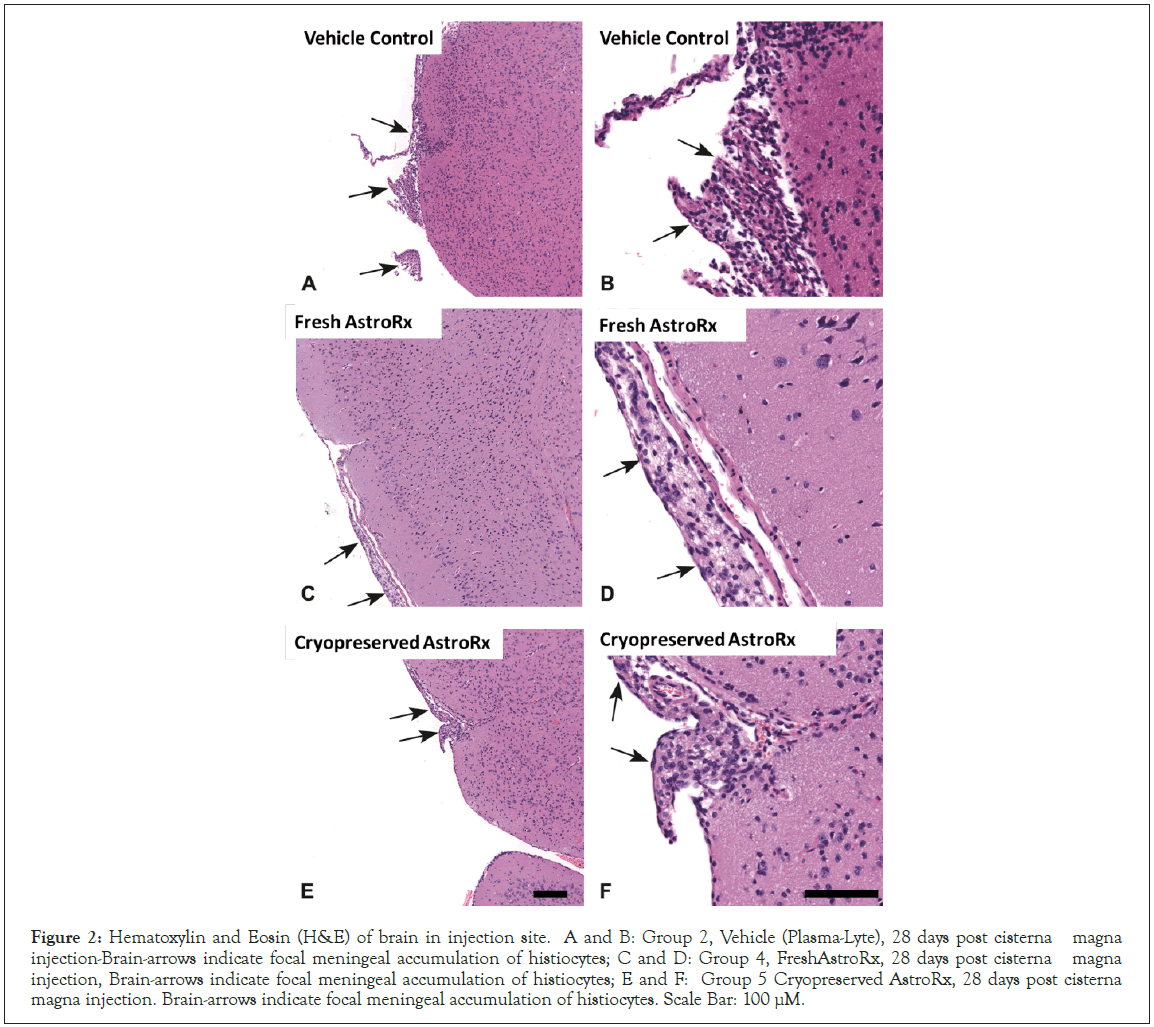
Figure 2: Hematoxylin and Eosin (H&E) of brain in injection site. A and B: Group 2, Vehicle (Plasma-Lyte), 28 days post cisterna magna injection-Brain-arrows indicate focal meningeal accumulation of histiocytes; C and D: Group 4, FreshAstroRx, 28 days post cisterna magna injection, Brain-arrows indicate focal meningeal accumulation of histiocytes; E and F: Group 5 Cryopreserved AstroRx, 28 days post cisterna magna injection. Brain-arrows indicate focal meningeal accumulation of histiocytes. Scale Bar: 100 µM.
Figure 3: Detection of fresh and cryopreserved AstroRx® cells 4 Weeks post Dosing. A and B: Positive Alu AstroRx (fresh) cells in brain; C and D: Positive Alu AstroRx (cryopreserved) in brain. Scale Bar: 1 mm (A), 500 µm (B) and 100 µm (C and D).
In view of the reported findings and under the conditions of this study, it can be concluded that a single injection to the cisterna magna to male and female NSG mice of cryopreserved AstroRx® (resuspended in plasmaLyte containing 0.23% DMSO and 0.62% HSA), as well as the Fresh AstroRx cells (in plasmaLyte) was not associated with significant neurological signs or major systemic adverse effects, and considered safe for use in a single administration. In additional the local and systemic effects of the two test items were comparable.
For a successful, clinical outcome of any cell therapy, the timely delivery of consistently reliable and effective cells to patient is critical. Significant difficulties arise when the clinics and hospitals are separated by distance and by time from the facilities where the cells are manufactured and prepared. In addition, the quality testing results (e.g., sterility, potency) of fresh cell product may be obtained after the cells were already administered. The development of a cryopreserved cell therapy product would allow the completion of more accurate longer sterility testing, the completion of potency results, simplify the process of manufacturing scaling, enable long term storage and transport, facilitate timing of therapy delivery to the patient, by that securing clinical objectives [2,5,34]. Here we report on the development of cryopreserved off-the-shelf cell product (a.k.a cryopreserved AstroRx®) that meet the release criteria that were defined for AstroRx® cells used for the clinical study [1]. AstroRx® cells are cryopreserved in physiological solution (plasmaLyte) supplemented with 3.5% DMSO and 9.3% Human Serum Albumin (HSA). To minimize manipulations steps at the clinical site the thawed cells are diluted in PlasmaLyte (1:15), which results with a final concentration of 0.23% DMSO and 5 × 106/ ml AstroRx® cells. The intended volume for injection is 20 ml, corresponding to the desired cell dose of 100 × 106 Astro® cells, cell dose that demonstrated high safety profile as well as meaningful clinical [1]. Important to note, that washing of DMSO from cryopreserved cells after thawing suffers from several disadvantages that should be avoided, this includes high cell lose, high risk for contamination and the need to have the process done in a certified clean rooms which are not abundantly present near the clinic.
Almost no information exists regarding the toxicological effects of DMSO following intrathecal injection to the CSF. Some predictions can be made for the exposure of the CNS to intended DMSO level. The average of total CSF volume in humans is generally quoted as approximately 130 ml [35,36], with a flow rate of 21-24 ml/h [37-39], which results with CSF turnover rate of 3-4 times a day [40]. Assuming the most stringent case of no distribution outside the CSF, an IT injection of AstroRx® contains a total of 47 mg of DMSO. Thus, the expected concentration of DMSO in the CSF following AstroRx® injection is estimated at ~0.035% (0.23% DMSO diluted in CSF 1:6.5). This concentration generates a safety margin of more than ~1100 from the concentration commonly used in most hematopoietic cell products injected IV (1 g/Kg bodyweight given IV divided by 0.85mg/Kg body weight for AstroRx®) [21]. In normal conditions, it is more likely that the steady state concentration will be even lower than the above calculations as DMSO crosses the blood brain barrier and its distribution across the entire body fluids is fast [41].
The safety of intrathecal injection of the cryopreserved AstroRx® cells and its vehicle control at the intended clinical use was confirmed here by the toxicological studies with up to 4 weeks follow up time. The toxicity of DMSO is reported to be transient and can be observed few hours till few days after exposure [42,43], thus, the animals were followed for a duration of a up to 4 week which allows the detection of any adverse event related to DMSO by IT administration used in AstroRx® drug product. In addition, the transient effect of DMSO by single injection is also supported by its short half-life and the rapid clearance of DMSO from rhesus monkeys [44]. Additionally, in previous preclinical safety study performed on AstroRx® cells, any adverse events related to AstroRx® cell therapy was already detected 4 weeks after intrathecal injection [26].
In our study, intrathecal injection of both vehicle and cell injected animals was associated with rare cases of minimal accumulations of meningeal histiocytes. Histiocytosis is a general name for a group of disorders or "syndromes" that involve an abnormal increase in the number of specialized white blood cells (usually macrophages) that are called histiocytes [45]. These lesions, were usually focal, located at the injection site, and were not associated with any damage to the adjacent brain tissue. Intrathecal injection to the CSF through the cisterna magna is a delicate procedure which might result with local damage to the CNS at the injection site, which in turn might stimulate histiocytosis [29]. Thus, this change is most probably related to reaction associated with the intrathecal administration procedure and not to any of the test compound components. Important to note, that IT injection in the clinics is performed through lumbar puncture, a standard procedure with high safety profile [46,47], which minimize the risks associated with AstroRx® cell injection.
In conclusion, a single IT administration of cryopreserved AstroRx®, an astrocyte cell-based therapy derived from embryonic stem cells, preserved its quality attributes and was found safe in comprehensive toxicology study. To our knowledge, this is the first time, that the toxicology profile of thawed cells (AstroRx®), injected IT together with low amount if DMSO, is demonstrated. To further determine the clinical effect of cryopreserved AstroRx® in ALS, additional powered, controlled clinical studies to evaluate repeated administration of AstroRx® is required.
MI, TS and MR conceived and designed the studies. ER, RD, DB, NK, RM, AN, and SGS performed the experiments. GK and IR were responsible for quality assurance. MI, TS, AN, and RM analyzed the data. MI and TS wrote the manuscript. TS, MI, NK, AN, and RM interpreted the data and reviewed the manuscript. All authors read and approved the final manuscript.
The study entitled: "Neurotoxicity Evaluation of Intrathecal Injection (into CSF) of DMSO at low concentrations in mice" was performed by SIA -Science in Action, Weizmann Science Park, Nes Ziona 70400 Israel. Under the approval of "The Israel Board for Animal Experiments" On April 2021. Ethics Committee Approval Number: IL-b18912-16.
The study entitled: "Assessment of toxicity, following a single injection to the Cisterna Magna in NSG mice" was performed under GLP conditions at Envigo CRS Israel on February 2022, under the approval of the National Council for Animal Experimentation. Ethics Committee Approval Number: IL-2112-132-4.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Kadimastem Ltd (affiliation 1) develops and manufactures the human embryonic stem cells derived astrocytes (AstroRx® cells) used for the preclinical toxicology studies. Authors related to affiliations 2 and 3 do not work at Kadimastem Ltd and served as the CROs (Contract Research Organization) for animal studies.
Funding from the Office of Chief Scientist, Ministry of Economy, Israel was awarded to Kadimastem Ltd. Award number: 77571. The funding body played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
The author declared no conflict of interest.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Sonnenfeld T, Rauchbach E, Downey R, Blumenkrants D, Hasson A, Kuperstein G, et al. (2023) Toxicity Studies on Intrathecal Injection of Low Dose of DMSO Used for Cryopreservation of Human Astrocytes in Mice. J Clin Toxicol. 13:553.
Received: 29-Nov-2023, Manuscript No. JCT-23-28318; Editor assigned: 01-Dec-2023, Pre QC No. JCT-23-28318 (PQ); Reviewed: 15-Dec-2023, QC No. JCT-23-28318; Revised: 22-Dec-2023, Manuscript No. JCT-23-28318 (R); Published: 29-Dec-2023 , DOI: 10.35248/2161-0495.24.13.553
Copyright: © 2023 Sonnenfeld T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.