Journal of Hepatology and Gastrointestinal disorders
Open Access
ISSN: 2475-3181
ISSN: 2475-3181
Research Article - (2019)Volume 5, Issue 2
Introduction: Hepatic Encephalopathy is one of the most common causes of hospitalization for cirrhotic patients. Lactulose has long been used as the standard therapy for the treatment of acute HE. This study evaluated the efficacy of PEG as compared with lactulose for the initial treatment of HE.
Aims and Objective: (1) To compare efficacy of PEG 3350 electrolyte solution over lactulose in patients admitted for hepatic encephalopathy. (2) To determine whether treatment with PEG will reduce duration of hospital stay, and whether PEG can be an effective additional treatment option for HE.
Material and methods: This prospective, randomized, comparative study was conducted in the Department of Gastroenterology Medical Trust hospital, Kochi–Kerala India, over a period of two year from May 2015–April 2017 following its approval by the Institutional ethical committee. 50 patients with cirrhosis and altered mental status attributed to HE were randomized to a standard lactulose protocol or a PEG protocol (25 in each group).
Interventions: Patients in the PEG group (n=25) received 2 L of PEG orally or via NG tube as a single dose over 4 hours. Patients in the lactulose group (n=25) received 20-30 g lactulose orally or via NG tube for 3 or more doses over 24 hours, or a single dose of 200 g lactulose via rectal tube. Grade of HE was determined prior to treatment and again at 24 hours using the Hepatic Encephalopathy Scoring Algorithm (HESA). After 24 hours, all patients received lactulose per the standard of care.
Main outcomes and measures: The primary end point was an improvement of 1 or more in HE grade at 24 hours, determined using the Hepatic Encephalopathy Scoring Algorithm (HESA), ranging from 0 (normal clinical and neuropsychological assessments) to 4 (coma). Secondary outcomes included time to HE resolution and overall length of stay.
Results: In our study, the gender distribution among two study groups showed a male predominant. Majority of HE patients were found in age group of 55-64 years. The most common underlying etiology of Liver Cirrhosis was Alcoholic liver disease 70%, Cryptogenic 14%, followed by Hepatitis C. The most common precipitant for Hepatic encephalopathy was GI Bleeding, followed by Constipation and Sepsis. The two groups were comparable in terms of baseline laboratory parameters. All patients were of Child Turcot Pugh (CTP) class C, with a Mean MELD score of 19.08 ± 2.23 in PEG group vs. 18.76 ± 2.36 in Lactulose group (p-value=0.625, NS). Majority of the patients were in grade 3 encephalopathy 58% (29/50), followed by grade 2 in 32% (16/50) at the time of presentation. A significant difference was seen between two groups in terms of mean change in grade of encephalopathy (HESA score) after 24 hours of therapy, with 1.00 ± 1.04 in PEG group compared to 1.76 ± 0.87 in lactulose group, with a significant p-value of <0.007. A significant difference between two groups in terms of mean time taken for complete resolution of hepatic encephalopathy, with 2.12 ± 0.52 days in PEG group compared to 3.76 ± 1.05 days in Lactulose group, with a significant p-value of <0.001, However there was no significant difference in length of hospital stay between two groups, with a mean hospital stay of 8.32 ± 1.77 days in PEG group compared to 8.28 ± 1.51 days in Lactulose group, with a (p-value=0.93). There was no significant difference in terms of mean change in serum ammonia level, serum Na+ and K+ after 24 hours, between the treatment groups. Overall, treatment regimens were similar in terms of tolerability, with the exception that in the lactulose arm, there was more bloating, while PEG patients experienced more of diarrheal symptoms.
Conclusions: PEG led to more rapid HE resolution than standard therapy, as compared with lactulose alone, the use of PEG alone during the first 24 hours of presentation worked better at improving symptoms of HE. The benefit beyond this time is less clear as both groups in this study received lactulose after 24 hours.
Hepatic encephalopathy; PEG; Lactulose; HESA
Hepatic Encephalopathy (HE) is a serious but potentially reversible disorder with a wide spectrum of neuropsychiatric abnormalities and motor disturbances that range from mild alteration of cognitive and motor function to coma and death [1]. It is a challenging complication of advanced liver disease and is estimated to occur in 30% to 45% of patients with liver cirrhosis and in 10% – 50% of patients with transjugular intrahepatic portosystemic shunts [2]. HE is often a serious sequela of chronic liver disease with significant morbidity, mortality and healthcare costs. In the United States HE accounts for 22,931 hospitalizations with an average stay of 8.5 days and a total cost of $64,108 per case [3].
Lactulose (beta-1, 4-galactosido-fructose) has been the standard of care for management of Hepatic encephalopathy for decades [4]. Lactulose is a non-absorbable synthetic disaccharide that consists of galactose and fructose linked by bond resistant to lactase. Lactulose is not absorbed by the small intestine but undergoes fermentation in the colon to yield short chain fatty acid, carbon dioxide, and hydrogen, with consequent lowering of fecal PH.
The mechanism of action of lactulose is multifactorial and is postulated to be the trapping of ammonium ions in the gut by organic acids released after bacteria metabolize lactulose or the removal of ammoniagenic organisms and/or replacement of these species with acidophilic bacteria lacking urease [5,6]. Others have suggested that inhibition of intestinal glutamine uptake and subsequent decreased ammonia genesis plays a role [7].
Polyethylene Glycol (PEG) is an iso-osmotic laxative that is metabolically inert and able to bind water molecules, thereby increasing intraluminal water retention. PEG is not metabolized by colonic bacteria. Ingestion of PEG leads to an increase in stool volume and softer stools, which may become liquid depending on the volume of PEG consumed [8].
PEG is being studied for the treatment of hepatic encephalopathy in patients with cir-rhosis and limited research has shown positive effects [9-12]. There are very few studies comparing lactulose and PEG in HE, and Indian data on this comparison is lacking. So in this study our aim was to determine whether PEG may represent an additional therapeutic option for treatment of patients with overt Hepatic encephalopathy [13,14].
Aims and objectives
To compare efficacy of PEG 3350 electrolyte solution over lactulose in patients with cirrhosis admitted for hepatic encephalopathy.
To determine whether treatment with PEG will reduces duration of hospital stay.
To determine whether PEG can be an effective additional treatment option for HE.
This single center prospective, randomized, comparative study was conducted in the Department of Gastroenterology Medical Trust hospital, Kochi, Kerala, India which is a tertiary care referral center. All Cirrhotic patients presenting to emergency department with altered mental status were eligible.
The Sample size for the present study was determined by the following formula n (for each sample)=(two sided) where Z1-α/ 2=1.96 (two sided, α=0.05) and Z1–β=0.84 (one sided) with power 80%, δ is equals to difference between two means (PEG and Lactose respectively) taken as 0.8, μ1=0.7, μ2=1.5 and σ1 and σ2 is equal to 0.93 and 1 respectively. The value comes to be 23 with 10 to 20% non-response; this can be taken as 25 in each group.
Inclusion criteria
Patients aged 18-80 years.
Both male and female subjects of all races and ethnicities.
Patients with liver cirrhosis of any cause.
Patients with any grade of hepatic encephalopathy [1-4].
Exclusion criteria
Patients with acute liver failure.
Patients with structural brain lesions (as indicated by computed tomography imaging if available and confirmed by neurological exam).
Patients with other causes of altered mental status (i.e. not meeting the definition of hepatic encephalopathy).
Patients with previous use of rifaximin or neomycin in past 7 days
Patients with suspected intestinal obstruction, recurrent vomiting.
Patients with pregnancy.
Patients with serum Sodium <125 mEq/L.
Patients receiving >1 dose of lactulose prior to enrollment.
Patients with uncontrolled infection and hemodynamic instability requiring vasopressors.
This prospective, randomized, comparative study was conducted in department of Gastroenterology Medical Trust Hospital, which is a 750 bedded Tertiary Care teaching hospital and referral center in Kochi, Kerala, India. The study was conducted over a period of two year from May 2015–April 2017, following its approval by the Institutional ethical committee of Medical Trust hospital. All the studied patients provided written informed consent through their Legally Authorized Representatives (LARs) prior to their participation.
Data collection
All patients presenting to emergency department with known cirrhosis and altered mental status were eligible to participate. For study purpose Hepatic Encephalopathy (HE) was defined as altered mental status with typical signs and symptoms, in the absence of an obvious cause of Altered Mental Status (AMS). The causes of cirrhosis were ascertained and precipitating factors were identified by two team members.
Data was collected from all enrolled patients on history, Physical examination and laboratory parameters. Clinical variables including adverse events and HESA score was calculated daily, until patient was discharged home or died during hospitalization.
Patient recruitment and participant randomization
When the diagnosis of hepatic encephalopathy was made, the study team was notified. The protocol stipulated that potential participants could be treated with a single dose of lactulose prior to randomization. Furthermore by design, the amount and route of administration were at the discretion of the first health care professional to administer care. After assessment of eligibility criteria, a member of the study team interviewed the patients LAR. If the LAR agreed to participate and consent provided, an opaque sealed envelope was opened. The envelope contained a computer generated number and treatment assignment. The random code sequence was blinded from the study investigators.
After patient enrollment and HESA score evaluation on admission, patients received either Polyethylene Glycol 3350 electrolyte solution (PEG) or standard of care therapy Lactulose, prescribed by treating physician. The standard controlled treatment consists of lactulose 20-30 g administered orally or by nasogastric tube (3 or more doses within 24 hours) or 200 g by rectal tube if oral intake is not possible or inadequate, at the discretion of treating physician. The study treatment consisted of 2 L of Polyethylene Glycol 3350 electrolyte solution (PEG) administered orally or via nasogastric tube, again at the discretion of treating physician. PEG was administered in a single dose over 4 hours.
After PEG administration, no lactulose was allowed for 24 hours, at which time the follow up HESA score was obtained. After 24 hours patients were allowed to receive lactulose as per the standard of care. Clinical variables including adverse events and HESA scores were collected daily until the patient refuses, discharged or dies during hospitalization.
HESA testing
We used HESA score to evaluate the severity of Hepatic encephalopathy. This scoring system is an objective instrument and is more widely adopted than West Haven Criteria. In order to avoid interpersonal variations HESA score (Table 1) at baseline and 24 hours afterwards were calculated by the same investigator. The 24 hour interval was chosen to minimize difference in circadian rhythm.
| HE grade | Clinical assessment | Neuropsychological assessment | HE grade determination |
|---|---|---|---|
| 4 | No eyes open | Not applicable | All 3 indicators present |
| No verbal response | |||
| No reaction to simple commands | |||
| 3 | Somnolence | Mental control | At least 3 indicators present, either clinical or neurophysiological |
| Confusion | |||
| Disoriented to place | |||
| Bizarre behavior/anger/rage | |||
| Clonus/rigidity/nystagmus/babinsky | |||
| 2 | Lethargy | Slow responses | at least 2 clinical and 3 neurophysiological indicators present |
| Disoriented to time | Anxiety | ||
| Slurred speech | Amnesia recent events | ||
| Hyperactive reflexes | Simple calculation | ||
| Inappropriate behavior | |||
| 1 | Sleep disorder | Complex calculation | At least 4 indicators present, either clinical or neurophysiological |
| Tremor | Construction ability | ||
| Shortened time span | |||
| Depression |
Table 1: Hepatic Encephalopathy Scoring Algorithm (HESA).
Primary outcome
Neurocognitive improvement of ≥1 in HE grade at 24 hour post admission determined using HE Scoring Algorithm (HESA) ranging from 0 (normal clinical and neuropsychological assessment) to 4 (coma).
Secondary outcomes
Time to resolution of HE.
Overall length of hospital stay.
A total of 172 cirrhotic patients (Figure 1) with hepatic encephalopathy were admitted in our Gastroenterology department from May 2015 to April 2017. Among them 50 patients who met the inclusion criteria were included in the study and were randomized into two groups, PEG group and Lactulose group with 25 patients in each group i.e., (25 patients in PEG group and 25 patients in Lactulose group). The reasons for exclusion were many like septic shock with MODS, severe hyponatremia, recurrent vomiting, consent not given, had received more than one dose of lactulose etc.
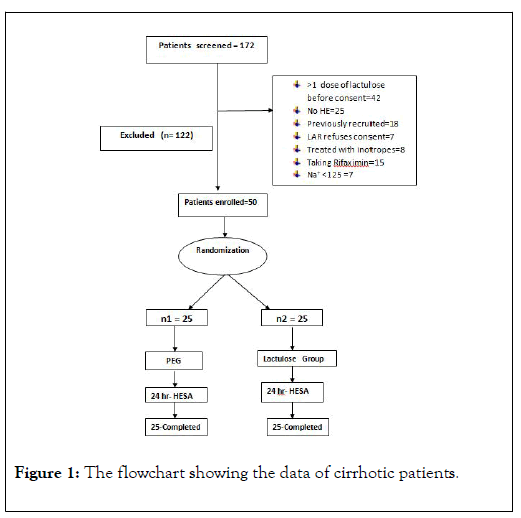
Figure 1: The flowchart showing the data of cirrhotic patients.
In our study, the gender distribution among two study groups showed, there was a male predominant population. Out of 50 patients 39 were males and 11 were females. In PEG group out of 25 patients, 20 (80%) were males and 5 (20%) were females as compared to Lactulose group, in which males were 19 (76%) and females were 6 (24%) with a p-valve of 0.733 (NS), (Figure 2).
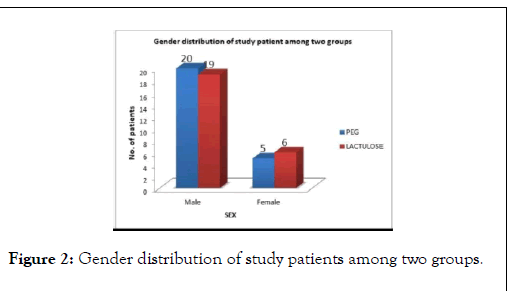
Figure 2: Gender distribution of study patients among two groups.
Majority of HE patients were found in age group of 55-64 years followed by 65-80. Mean age was 62.12 years with a SD of ±5.93 in PEG group compared to 60.48 years with SD of ±8.45 in Lactulose group (p-value=NS), (Figure 3).
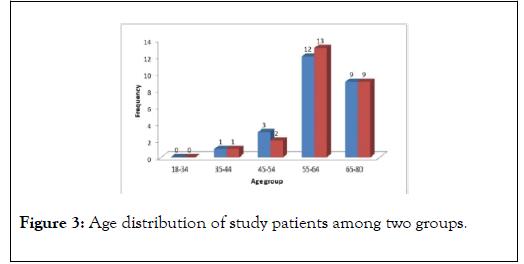
Figure 3: Age distribution of study patients among two groups.
The most common underlying etiology of Liver Cirrhosis in our study population was Alcoholic liver disease in 70% (35/50) patients, Cryptogenic in 14% (7/50) patients, followed by Hepatitis C and Hepatitis B 4% (2/50) in each respectively (Figure 4).
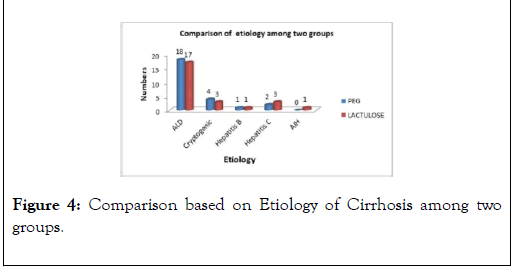
Figure 4: Comparison based on Etiology of Cirrhosis among two groups.
In our study population, the most common precipitant for Hepatic encephalopathy was GI Bleeding, 42% (21/50),followed by Constipation 26% (13/50) and Sepsis in 18% (9/50) patients, (Figure 5).
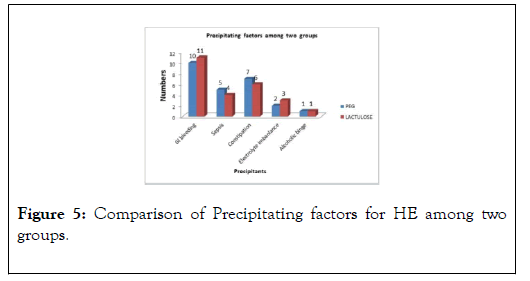
Figure 5: Comparison of Precipitating factors for HE among two groups.
The most commonly encountered comorbidities in our study population were (Hypertension+CAD) in 42% (21/50), followed by BHP 18% (9/50), Diabetes Mellitus 14% (7/50), and Obesity in 14% (7/50) patients (Figure 6).
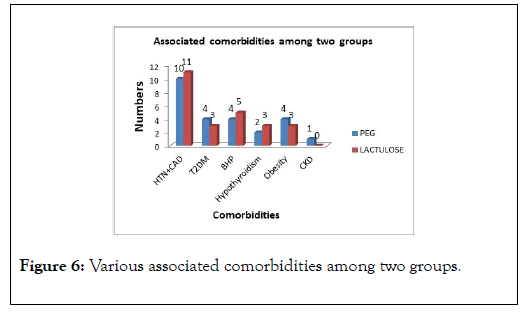
Figure 6: Various associated comorbidities among two groups.
The two groups were comparable in terms of baseline laboratory parameters, like WBC count, Hemoglobin, Platelets, Bilirubin, Albumin, INR, Urea, Creatinine, serum Sodium and Potassium (p-value=NS) (Table 2).
| Laboratory parameters | All patients | PEG | Lactulose | p-value | |||
|---|---|---|---|---|---|---|---|
| (n=50) | (n=25) | (n=25) | |||||
| Mean | S.D | Mean | S.D | Mean | S.D | ||
| WBC count*106/L | 9.49 | 7.68 | 10.38 | 1.03 | 8.6 | 3.54 | 0.145 |
| Hemoglobin | 9.26 | 1.8 | 9.25 | 1.76 | 9.26 | 1.89 | 0.89 |
| Platelet count*103/µL | 45.42 | 19.69 | 46.64 | 19.99 | 44.2 | 19.73 | 0.66 |
| T. Bilirubin | 4.01 | 1.554 | 3.96 | 1.56 | 4.07 | 1.55 | 0.805 |
| Albumin | 2.58 | 0.5 | 2.56 | 0.5 | 2.6 | 0.52 | 0.808 |
| INR | 1.66 | 0.29 | 1.68 | 0.32 | 1.64 | 0.28 | 0.675 |
| Urea | 53.04 | 10.84 | 53.24 | 10.84 | 52.84 | 11.07 | 0.898 |
| Creatinine | 1.41 | 0.29 | 1.42 | 0.34 | 1.4 | 0.25 | 0.852 |
| Sodium | 136.64 | 5.18 | 135.96 | 4.01 | 137.31 | 6.15 | 0.362 |
| Potassium | 3.69 | 0.63 | 3.72 | 0.57 | 3.51 | 0.68 | 0.252 |
Table 2: Comparison of baseline laboratory parameters among two groups.
All patients were of Child Turcot Pugh (CTP) class C, with a Mean MELD score of 19.08±2.23 in PEG group vs. 18.76±2.36 in Lactulose group respectively with a p-value of 0.625, (Figure 7).
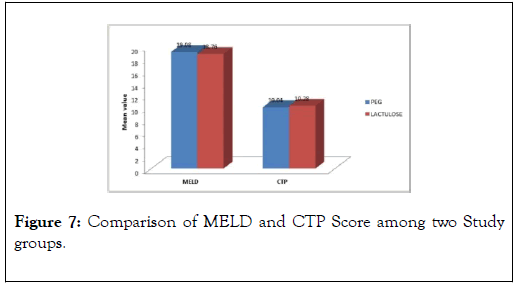
Figure 7: Comparison of MELD and CTP Score among two Study groups.
Majority of the patients were in grade 3 encephalopathy 58% (29/50), followed by grade 2 in 32% (16/50) at the time of presentation. There was no significant difference between two groups in terms of HESA grade of encephalopathy at presentation (p-value=0.81) (Table 3).
| Baseline HESA Score | All patient | PEG | Lactulose | p-value | |||
|---|---|---|---|---|---|---|---|
| No | % | No | % | No | % | ||
| Grade IV | 5 | 10 | 3 | 12 | 2 | 8 | 0.64 |
| Grade III | 29 | 58 | 13 | 52 | 16 | 64 | 0.39 |
| Grade II | 16 | 32 | 9 | 36 | 7 | 28 | 0.27 |
| Mean±SD | 2.76±0.66 | 2.8±0.57 | 0.81 | ||||
Table 3: Showing grade of hepatic encephalopathy at baseline.
A significant difference between two groups in terms of mean change in grade of encephalopathy (HESA score) after 24 hours of therapy, with 1.00±1.04 in PEG group compared to 1.76±0.87 in lactulose group, with a significant p-value<0.007, (Tables 4 A and B).
| A) 24 hrs HESA Score | All patient | PEG | Lactulose | p-value | |||
|---|---|---|---|---|---|---|---|
| No | % | No | % | No | % | ||
| Grade III | 8 | 16 | 3 | 12 | 5 | 20 | 0.44 |
| Grade II | 15 | 30 | 4 | 16 | 11 | 44 | 0.03 |
| Grade I | 15 | 30 | 8 | 32 | 7 | 28 | 0.76 |
| Grade 0 | 12 | 24 | 10 | 40 | 2 | 8 | <0.001 |
| Mean±SD | 1.04 | 1.76±0.87 | 0.007 | ||||
Table 4(A): Showing grade of hepatic encephalopathy at =24 hrs.
| B) Change in HESA score at 24 hours from presentation | PEG | Lactulose | p-value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| 1 | 1.04 | 1.76 | 0.87 | <0.007 | |
Table 4(B): Showing grade of hepatic encephalopathy at ˃24 hrs.
A significant difference between two groups in terms of mean time taken for complete resolution of hepatic encephalopathy, with 2.12±0.52 days in PEG group compared to 3.76±1.05 days in Lactulose group, with a significant p-value<0.001 (Table 5) (Figure 8).
| Time taken for complete resolution of HE ( in days) | PEG | Lactulose | p-value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| 2.12 | 0.52 | 3.76 | 1.05 | <0.001 | |
Table 5: Comparison between two groups in terms of complete resolution of Hepatic encephalopathy.
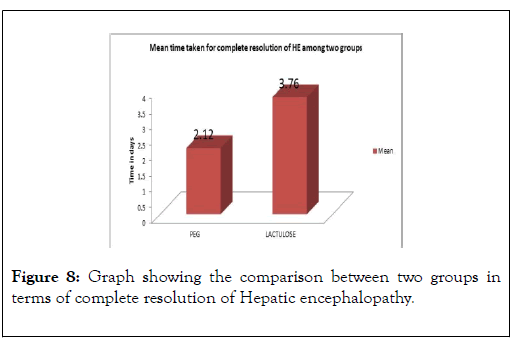
Figure 8: Graph showing the comparison between two groups in terms of complete resolution of Hepatic encephalopathy.
However there was no significant difference in length of hospital stay between two groups, with a mean hospital stay of 8.32±1.77 days in PEG group compared to 8.28±1.51 days in Lactulose group, with a p-value of 0.93 (Figure 9).
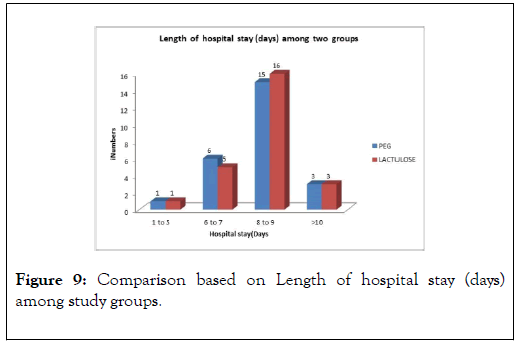
Figure 9: Comparison based on Length of hospital stay (days) among study groups.
There was no significant change in blood level of ammonia after 24 hours between the two groups. In PEG group, the mean serum NH3 level at baseline was 74.17±15.9 compared to mean NH3 of 70.06±16.7 at 24 hours after presentation, with p-value of 0.40. Similarly the mean serum NH3 level at baseline was 72.32±18.27 compared to 65.27±18.6 at 24 hours of presentation in Lactulose group, with p-value of 0.18 (Table 6).
There was no significant difference in terms of mean change in serum sodium (Na+) after 24 hours, between the treatment groups with p-value of 0.89 in PEG group compared to Lactulose group with a p-value of 0.55 (NS) (Table 7).
| 24 hours change in Serum ammonia level | PEG | Lactulose | ||||
|---|---|---|---|---|---|---|
| NH3 at day 0 (baseline) | NH3 at day 1 (≥24 hours) | p-value | NH3 at day 0 (baseline) | NH3 at day 1 (≥24 hours) | p- value | |
| Mean±SD | Mean±SD | Mean±SD | Mean±SD | 0.18 | ||
| 74.17±15.9 | 70.06±16.7 | 0.4 | 72.32±18.27 | 65.27±18.6 | ||
Table 6: Comparison of 24 hrs change in serum Ammonia level among two groups.
| 24 hours change in Serum Sodium level | PEG Group | Lactulose Group | ||||
|---|---|---|---|---|---|---|
| Na+ at day 0 (baseline) | Na+ at day 1 (≥24 hrs) | p-value | Na+ at day 0 (baseline) | Na+ at day 1 (≥24 hrs) | p-value | |
| Mean±SD | Mean±SD | Mean±SD | Mean±SD | 0.55 | ||
| 135±4.01 | 134.85±4.2 | 0.89 | 137.31±6.15 | 136.31±5 | ||
Table 7: Comparison of 24 hours change in serum Sodium (Na+) level among two groups.
Also there was no significant change in mean serum Potassium (K+) after 24 hours between the treatment groups with p-value of 0.70 in PEG group, as compared to Lactulose group with p-value of 0.71 (Table 8).
| 24 hours change in Serum Potassium level | PEG Group | Lactulose Group | ||||
|---|---|---|---|---|---|---|
| K+ at day 0 (baseline) | K+ at day 1 (=24 hrs) | p-value | K+ at day 0 (baseline) | K+ at day 1 (=24 hrs) | p-value | |
| Mean±SD | Mean±SD | Mean±SD | Mean±SD | 0.71 | ||
| 3.72±0.57 | 3.66±0.56 | 0.7 | 3.51±0.68 | 3.44±0.69 | ||
Table 8: Comparison of 24 hours changes in serum Potassium level among two groups.
The major strengths of our study were its innovative approach and to the best of our knowledge, it’s the first of its kind from our part of world. PEG preparations are widely available, commonly used, inexpensive and safe osmotic laxative. One potential benefits of using PEG for overt HE is that, the improvement in grade of encephalopathy (HESA score) after 24 hours was significantly rapid and early in PEG group. HE resolution was shown to be significantly more rapid in the PEG group. This could potentially result in a decrease in the hospital stay and cost of therapy
Adverse events
Both Polyethylene Glycol 3350 electrolyte solution and lactulose therapy were considered safe therapies with no definitive treatment related adverse events.
Two patients in Lactulose group and one patient in PEG group have died. All of the patients who died had advanced liver disease (MELD>28 and CTP class C) with septicemia, developed multi-organ failure and died. None of the deaths was related to therapy (PEG/Lactulose).
Overall, treatment regimens were similar in terms of tolerability, with the exception that in the lactulose arm, there was more bloating, while PEG patients experienced more of diarrhoeal symptoms.
The possibility that treatment may alter electrolyte levels in the follow–up period was also assessed. There was no significant change in serum electrolytes level among two groups at 24 hours after presentation of hepatic encephalopathy (Tables 7 and 8).
Statistical methods
The statistical analysis was performed by IBM SPSS Statistics 20 version and Microsoft Excel 2007 version. A categorical variable was described as frequency and percentage. Continues variable were described as Mean±SD. Pearson Chi-square test fisher’s exact test was used to find the association between categorical variable. Student t-test was used to check the difference in mean values of the variable between two groups.
The major strength of our study was its innovative approach and to the best of our knowledge, it’s the first of its kind from our part of world. PEG preparations are widely available, commonly used, inexpensive and safe osmotic laxative.
The results of this study indicate that bowel cleansing with PEG, normally used for colonoscopy preparation, is a safe, rapid, and effective immediate treatment strategy for patients presenting with acute overt HE. When compared with lactulose, the current standard treatment for overt HE, objective measures of HE in the first 24 hours, improved significantly faster in those receiving PEG. By accelerating improvement in mentation, initial treatment with PEG has the potential to not only shorten hospitalizations, but also permit health care professionals to concentrate on managing the precipitating factors and identifying other causes of metabolic encephalopathy that may be present.
Rahimi et al. [9], first time used PEG electrolyte solution in comparison to lactulose in patients with acute overt hepatic encephalopathy and found it to be superior to lactulose in improving hepatic encephalopathy. Our study also demonstrates that PEG 3350 Electrolyte solution is more effective than lactulose in improvement of hepatic encephalopathy.
The gender distribution among two study groups in our study showed, there was a male predominant population. Out of 50 patients 39 were males and 11 were females. The male predominant population in this study was likely due to the risk of chronic alcohol use as a cause of chronic liver disease which was consistent with other studies done by Rahimi et al. [9] and Naderian et al. [10] respectively.
In our study, majority of HE patients were found in age group of 55-64 years followed by 65-80 years. Our study group had advanced age with a Mean age of 62.12 ± 5.93 years in PEG group and 60.48 ± 8.45 years in Lactulose group (p-value=NS), as compared to Rahimi et al. [9], where mean age was 56 ± 11 in PEG group and 56 ± 7 in Lactulose group. In a study done by Naderian et al. [10], the mean age was 53.57 ± 11.61 yrs in (PEG +Lactulose) group compared to 59.63 ± 9.27 in Lactulose group alone.
The most common underlying etiology of Liver Cirrhosis in our study population was Alcoholic liver disease in 70% (35/50) patients, Cryptogenic in 14% (7/50) patients, followed by Hepatitis C and Hepatitis B 4% (2/50) each respectively, as compared to the study done by Rahimi et al. [9], where the most common cause of liver cirrhosis was alcohol in 38%, Hepatitis C in 34%, followed by Cryptogenic 24%, and Hepatitis B in 4%. Naderian et al. [10] reported Hepatitis B as their main etiological factor for liver cirrhosis in 30% (12/40), followed by Cryptogenic 27.5% (11/40), Hepatitis C 22.5% (9/40) and alcohol in 5% (2/40) patients respectively. Reason for this difference was alcohol consumption is very common in our society and easily available
Most patients admitted with HE had more than one precipitating factor. In our study population, the most common precipitant for Hepatic encephalopathy was GI Bleeding, 42% (21/50) patients, followed by Constipation, 26% (13/50) and Sepsis in 18% (9/50) patients, which was almost similar to the study done by Naderian et al. [10].
In our study population, we have observed a higher MELD score with a mean MELD score of 18.92 ± 2.28, (MELD score of 19.08 ± 2.23 in PEG group vs. 18.76 ± 2.36 in Lactulose group, p-value=0.625) and higher WBC count (mean WBC count 9.46 ± 7.68) at admission, compared to Rahimi et al. [9] in which mean MELD score was 17 ± 5 (MELD score of 17 ± 5 in PEG group vs. 17 ± 6 in lactulose group) and mean WBC count of 6.2 ± 2.6 at baseline. All patients in our study population were of Child Turcot Pugh (CTP) class C, with (mean CTP score=10.16 ± 1.85) which was in accordance with the study done by Rahimi et al. [9] where (mean CTP score=10 ± 2).
In our study we have observed, the improvement in grade of encephalopathy (change in HESA score) at 24 hours among two study groups after treatment was significant in PEG group (mean HESA score=1.00 ± 1.04 in PEG group vs. 1.76 ± 0.87 in Lactulose group) and was statistically significant with a pvalue< 0.007, which was similar to the study conducted by Rahimi et al. [9] (p-value of 0.002). A similar kind of study was performed by Naderian et al. [10], in which he observed significant improvement in 24 hrs HESA score grade in (PEG +Lactulose) group compared to Lactulose group alone, and was statistically significant with a p-value of 0.04 respectively. Reasons for this may be that PEG electrolyte solution is more powerful cathartic agent compared to lactulose and electrolyte solution might prevent dyselectrolemia which may contribute to improvement in encephalopathy.
A significant difference between two groups in terms of Mean time taken for complete resolution of hepatic encephalopathy, with 2.12 ± 0.52 days in PEG group compared to 3.76 ± 1.05 days in Lactulose group, with a significant p-value<0.001, compared to Rahimi et al. [9] study, where the median time to HE resolution was 1 day in patients receiving PEG compared with 2 days in those receiving lactulose with (p-value of 0.01).
A significant difference in our study compared to Rahimi et al. [9] study was the dose of PEG electrolyte solution which was about half of the dose used in study. Reason for decreasing the dose was that, our body-structure is significantly different than the Americans (In Indian population average weight is 57.7 kg and average height is 166.3 cm in males, 153 cm in females compared to US population where average weight is 80.7 kg and average height 175.5 cm in males and 162.1 cm in females) respectively.
Another significant difference in our study compared to study was that, there was no significant difference in length of hospital stay between two groups, with a mean hospital stay of 8.32 ± 1.77 days for PEG group and 8.28 ± 1.51 days for Lactulose group (p-value=0.93), compared with Rahimi et al. [9] study where mean duration of hospital stay was 4 ± 3 days in PEG group versus 8 ± 12 days in lactulose group. Naderian et al. [10], also observed a significant different in length of hospital study (mean hospital stay 6.8 ± 0.5 days in (PEG+Lactulose) vs. 8.9 ± 0.7 days in Lactulose group with p-value of 0.03. In view of advanced age, high MELD score and high WBC count, multiple precipitating factors for hepatic encephalopathy in individual patients and many patients of sepsis, may the reason for longer hospital stay.
We could not properly explain why quick improvement in hepatic encephalopathy in PEG group did not convert into shorter hospital stay for PEG group patients, with possible reason may be treatment of other complications of cirrhosis like SBP, variceal bleed and treatment of precipitating factors like sepsis took long time to improve.
There was no significant change in blood level of ammonia after 24 hours between the treatment groups. In PEG group, the mean serum NH3 level at baseline was 74.17 ± 15.9 compared to mean NH3 of 70.06 ± 16.7 at 24 hours after presentation, with p-value of 0.40 (NS). Similarly the mean serum NH3 level at baseline was 72.32 ± 18.27 compared to 65.27 ± 18.6 at 24 hours in Lactulose group, with p-value of 0.18 (NS), and was similar to the study done by Naderian et al. [10] (p-value=0.858, NS). Rahimi et al. [9] observed in his study, that the 24-hour difference in ammonia was greater in the lactulose group (i.e., overall lower ammonia level) than in the PEG group with pvalue of 0.03.
In our study, we have observed that PEG could improve hepatic encephalopathy more rapidly than lactulose in first 24 hours of therapy, but there were no significant differences in blood level of ammonia between the two groups. To the best of our knowledge, ammonia or better to say NH3 (not NH4+ ) plays the major role in altered mental status in cirrhotic patients, because only NH3 can easily pass over blood brain barrier [11]. If we measured the amount of free NH3 instead of total blood level of ammonia, we could probably find a difference between the two treatment groups. It is noteworthy that PEG causes a mild metabolic acidosis, resulting in increased NH4+ level and decreased NH3. These chemical changes might explain the effect of PEG in rapid improvement of altered mental status without any significant change in ammonia level [11,12]. In addition, some other studies have challenged the use of blood ammonia as an indicator for severity of hepatic encephalopathy, especially in grade 0 to grade 2 encephalopathy [13-14].
In our study, there was no significant difference in terms of mean change in serum sodium (Na+) after 24 hours of treatment, between the treatment groups with p-value of 0.89 in PEG group, compared to Lactulose group with a p-value of 0.55 (NS), which was in accordance with the study done by Naderian et al. [10] and Rahimi et al. [9] respectively.
Also there was no significant change in mean serum Potassium (K+ ) after 24 hours of therapy between the treatment groups with p-value of 0.70 in PEG group, compared to Lactulose group with p-value of 0.71, and was similar with the study done by Naderian et al. [10], and Rahimi et al. [9], respectively.
Overall, treatment regimens were similar in terms of tolerability, with the exception that in the lactulose arm, there was more bloating, while PEG patients experienced more of diarrheal symptoms, and this was similar with the study done by Rahimi et al. [9].
It is from a single center and thus may not be generalized to other centers.
The sample size in our study was relatively small, and this might not be representative of all India.
3) This study could not be blinded.
4) Only short term outcomes were studied.
When compared with lactulose, the current standard treatment for overt HE, objective measures of HE in the first 24 hours, improved significantly faster in those receiving PEG. By accelerating improvement in mentation, initial treatment with PEG has the potential to not only shorten hospitalizations, but also permit health care professionals to concentrate on managing the precipitating factors and identifying other causes of metabolic encephalopathy that may be present.
Since our study was small, single center based, there may be a need for multicentric, large trials to further confirm its efficacy.
Larger and multicenter trials with different doses of PEG, should be performed to make PEG as a routine treatment for patients with cirrhosis and HE.
Study comparing, combination of (PEG+Lactulose) versus lactulose alone needs to be undertaken to determine whether PEG can be an effective additional treatment option for HE.
Citation: Raja W, Jan R, Sebastian B, Mathai SK, Ashfaq (2019) To Compare the Effect of Polyethylene Glycol vs. Lactulose in the Treatment of Overt Hepatic Encephalopathy. J Hepatol Gastroint Dis 5:166. doi: 10.35248/2475-3181.5.166
Received: 30-Apr-2019 Accepted: 10-May-2019 Published: 17-May-2019
Copyright: © 2019 Raja W, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.