Journal of Proteomics & Bioinformatics
Open Access
ISSN: 0974-276X
ISSN: 0974-276X
Research Article - (2023)Volume 16, Issue 3
Background: Chronic Hepatitis B (CHB) infection and the Hepatitis B virus X protein (HBx) are major risk factors associated with Hepato Cellular Carcinoma (HCC). In CHB infection, HBx induces mitochondrial dysfunction, exhaustion and impaired function in hepatocytes. Restoring hepatocyte health along with reduction in virus replication could be an ideal treatment for CHB. Thiourea derivatives are well known for their antiviral properties, though their effect on mitochondrial and/or hepatocyte health remains obscure. This study focuses on the repurposing of thiourea derivatives (DSA-00, DSA-02 and DSA-09) for hepatocyte replenishment.
Methods: HepG2.2.15 cells were treated with thiourea derivatives, alongside Entecavir (ETV). HBV DNA, RNAs and antigens, measured by qPCR, Quantitative Reverse Transcriptase Polymerase Chain Reaction (RT-qPCR) and Enzyme-Linked Immunosorbent Assay (ELISA), respectively. Followed by proteomic analysis by Weighted Protein Co-expression Network Analysis (WPCNA) and Fluorescence Activated Cell Sorting (FACS) used for the validation of results.
Results: The proteomics analysis showed both DSA-00 and ETV were enriched with proteins associated with antiviral responses. In addition, DSA-00 additionally showed an increase in proteins linked to mitochondrial response. Whereas DSA-02 exhibited associations with the innate immune system and citric acid cycle and DSA-09 displayed pathways similar to DSA-00 and ETV. The treated groups exhibited an enhanced bio-energetic and antiviral response as compared to the untreated group. FACS analysis revealed the restoration of exhausted hepatocytes by thiourea derivatives through targeting mitochondria.
Conclusion: Our findings suggest that thiourea derivatives hold potential as a novel therapeutic agent that seems to restore mitochondrial health along with an anti-viral response in CHB.
Antiviral; Thiourea derivatives; Hepatitis B virus; Chronic hepatitis B infection; Mitochondria dysfunction; Exhausted hepatocytes
Chronic Hepatitis B (CHB) infection is caused by Hepatitis B Virus (HBV) that lasts for more than six months and leading to long-term liver disease. Currently, 257 million people around the world had a CHB infection [1,2]. CHB often leads to major problems with the liver, such as cirrhosis, liver failure and liver cancer. As the illness persists longer, there is more liver inflammation and damage, with likelihood of aggravation of these issues [3]. CHB can also alter mitochondrial and Endoplasmic Reticulum (ER) functions, which can contribute to liver damage and disease progression [4,5]. Patients with CHB show higher levels of mitochondrial DNA (mtDNA) in their serum than healthy, indicating increased mitochondrial dysfunction and damage [6]. Alterations in mitochondrial morphology and their membrane potential, metabolism and biogenesis have been reported in the HBV-infected hepatocytes [7,8]. CHB has also been linked to ER stress and its response was found to be activated in liver biopsies from patients with persistent HBV infection and this was linked to increased liver fibrosis and inflammation [9,10]. ER stress and dysfunction of mitochondria are associated to the progression to cirrhosis and end stage Hepatocellular Carcinoma (HCC) [11]. Several factors have been implicated in the development of HBV infection, including the HBx oncoproteins, immune-mediated death of HBV-infected hepatocytes and liver regeneration [12]. HBx regulates viral replication, viral and host genes transcription, various cell signaling activities, ubiquitin-proteasome machinery, cell cycle progression and programmed cell deaths etc [13]. The regulatory protein HBx, which plays an essential role in the viral life cycle, is encoded by the 0.7 kb RNA of HBV [14]. HBV is a member of the Hepadnaviridae family and has a partially double-stranded DNA genome of 3.2 kb. Its genome contains four Open Reading Frames (ORFs), which encode 3.5, 2.4, 2.1 and 0.7 kb RNAs and are translated into seven different proteins. The 3.5 kb RNA is responsible for encoding the core protein (HBcAg) and the pre-core protein (HBeAg), while the 2.4 and 2.1 kb RNAs encode the Small (S), Medium (M) and Large (L) surface proteins. Previous research suggests that the regulatory protein HBx may be responsible for various effects attributed to HBV 13. HBx induced aberrant aggregation of mitochondrial components near the nucleus periphery triggered by HBx-driven p38 Mitogen-Activated Protein Kinase (MAPK), which may eventually lead to cell deaths [15]. HBx damage mitochondria via interaction with Voltage-Dependent Anion Channel (VDAC3), which is known as mitochondrial porins and forms pores in mitochondrial outer membranes [16] and interaction alters mitochondrial transmembrane potential, resulting in the formation of Reactive Oxygen Species (ROS) and cellular ATP/ADP ratio [17]. Furthermore, HBx can suppress the expression of nucleus-encoded genes involved in mitochondrial beta-oxidation of fatty acids, resulting in a low level of cellular ATP due to energy source deficit. HBx-induced Raf-1 kinases pathway or the apoptosis regulator Bcl-2-Associated X protein (BAX) are translocated to mitochondria by HBx, resulting in hepatic cell proliferation or apoptosis, respectively [6]. HBV-infected cells synthesize a lot of surface proteins in the Endoplasmic Reticulum (ER), disrupting ER homeostasis and causing ER stress [10]. CHB patient’s biopsies reported with enlarge ER due to surface proteins (preS1 and preS2). ER stress or HBx can trigger autophagy [18]. These findings imply that HBx-induced mitochondrial dysfunction and ER stress could be contributed to hepatocellular carcinoma pathogenesis, including proliferation, metastasis and other features of carcinogenesis. Biological networks have been used as a powerful approach for identifying novel dysregulated pathways driving molecular pathology. Weighted Protein Correlation Network Analysis (WPCNA) is a multi-protein study that identifies groupings of proteins that are frequently altered and orchestrates them into modules without relying on a prior specified proteins setsor pathways. WPCNA is a useful technique for determining how networks of proteins and phenotypes are related [19].
Thiourea and its derivatives have been used therapeutically as antioxidants [20], anti-inflammatory drugs, anti-cancer [21], fungicides [22], as well as anti-viral [23,24]. Recently, a novel thiourea compound DSA-00 (IR-415) was identified by high-throughput screening of the May bridge library that suppresses HBV replication like current antivirals [23]. The present study is focused on the analysis of proteome alteration in cell (HepG2.2.15) under the influence of currently used anti-viral drugs in comparison to thiourea derivatives in order to identify key molecular changes linked to mitochondrial dysfunction and antiviral activity. We validated the proteomic finding of mitochondrial health using flow cytometry data with the ultimate goal of identifying whether repurposed thiourea derivatives could be improved mitochondrial and hepatocyte health.
Cell culture and reagents
HepG2.2.15 cells, which have integrated two copies of HBV genome, used in this study, were grown in Dulbecco Modified Eagle Medium (DMEM, Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Gibco of Invitrogen), GlutaMax, 10 mM HEPES, 100 unit/ml penicillin, 100 µg/ml streptomycin and 10% FBS at 37°C in a humidified atmosphere containing 5% CO2. DSA-00, DSA-02, DSA-09, Entecavir (ETV, Gilead Sciences) and Ascorbic Acid (Vitamin C, Sigma-Aldrich). All compounds were dissolved in dimethyl sulfoxide and stored at -20°C or -80°C until used.
Real-time PCR and reverse transcription-PCR
The cDNA from extracted total RNA from cells using SuperScriptIII (Invitrogen), followed by RT-qPCR/qPCR using PowerUP SYBR Green PCR Master Mix (Applied Bio systems). HBV DNA and pre-genomic RNAs were measured using the primer sets of HBV DNA-F CCTAGTAGTCAGTTATGTCAAC and HBV DNA-R TCTATAAGCTGGAGGAGTGCGA by qPCR and RT-qPCR. HBV total RNA was measured by RT-qPCR using set of primers (HBV RNA-F ACCGACCTTGAGGCATACTT and HBV RNA-R GCCTACAGCCTCCTAGTACA). The PCR was performed at 50°C for 2 minutes, 94°C for 10 minutes and 50 cycles of 94°C for 15 seconds and 60°C for 1 minute.
Enzyme-Linked Immunosorbent Assay
Hepatitis B Surface Antigens (HBsAg) and Hepatitis B e Antigens (HBeAg) were measured in cell culture supernatant via Enzyme-Linked Immunosorbent Assay (ELISA) kits followed the kit manufacturer's instructions (www.immunotag.com © 2018 Geno Technology Inc., USA. All Rights Reserved)
Proteomics analysis
Untargeted proteomic analysis of cell culture samples was performed using Ultra-High Performance Liquid Chromatography (UHPLC) coupled with High Resolution Tandem Mass Spectrometry (HRMS/MS) [25]. HepG2.2.15 cells (1x105), were seeded in six well plates. Following day, cells were treated with 10 µM of thiourea derivatives, ETV and Vitamin C. After 24 h, cells were trypsinized and collected by centrifugation at 5000 Revolutions per Minute (RPM). The cells pellet was then re-suspended in RadioImmuno Precipitation Assay (RIPA) lysis buffer to isolate total proteins. The detailed protocol for the untargeted proteomics analysis are provided in the Supporting Information (SI).
MTR and MTG HepG2.2.15 cell staining and flow cytometry analysis
MitoTracker Red (MTR) CM-H2XROS and MitoTracker Green (MTG) staining were done according to the manufacturer's instructions (Molecular Probes). Detailed methods are provided in the SI (Figures 1A, 2A).
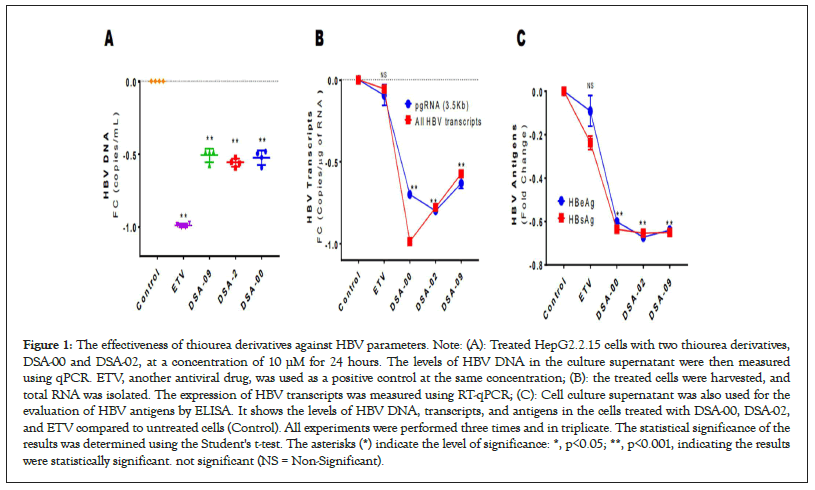
Figure 1: The effectiveness of thiourea derivatives against HBV parameters. Note: (A): Treated HepG2.2.15 cells with two thiourea derivatives, DSA-00 and DSA-02, at a concentration of 10 μM for 24 hours. The levels of HBV DNA in the culture supernatant were then measured using qPCR. ETV, another antiviral drug, was used as a positive control at the same concentration; (B): the treated cells were harvested, and total RNA was isolated. The expression of HBV transcripts was measured using RT-qPCR; (C): Cell culture supernatant was also used for the evaluation of HBV antigens by ELISA. It shows the levels of HBV DNA, transcripts, and antigens in the cells treated with DSA-00, DSA-02, and ETV compared to untreated cells (Control). All experiments were performed three times and in triplicate. The statistical significance of the results was determined using the Student's t-test. The asterisks (*) indicate the level of significance: *, p<0.05; **, p<0.001, indicating the results were statistically significant. not significant (NS = Non-Significant).
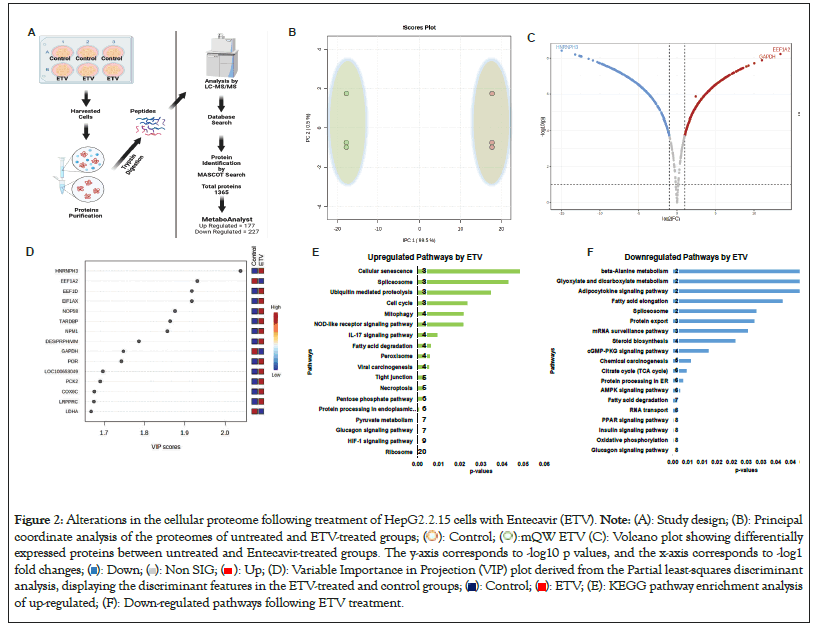
Figure 2: Alterations in the cellular proteome following treatment of HepG2.2.15 cells with Entecavir (ETV). Note: (A): Study design; (B): Principal
coordinate analysis of the proteomes of untreated and ETV-treated groups;  (C): Volcano plot showing differentially
expressed proteins between untreated and Entecavir-treated groups. The y-axis corresponds to -log10 p values, and the x-axis corresponds to -log1 fold changes;
(C): Volcano plot showing differentially
expressed proteins between untreated and Entecavir-treated groups. The y-axis corresponds to -log10 p values, and the x-axis corresponds to -log1 fold changes;  (D): Variable Importance in Projection (VIP) plot derived from the Partial least-squares discriminant
analysis, displaying the discriminant features in the ETV-treated and control groups;
(D): Variable Importance in Projection (VIP) plot derived from the Partial least-squares discriminant
analysis, displaying the discriminant features in the ETV-treated and control groups;  (E): KEGG pathway enrichment analysis
of up-regulated; (F): Down-regulated pathways following ETV treatment.
(E): KEGG pathway enrichment analysis
of up-regulated; (F): Down-regulated pathways following ETV treatment.
Statistical and correlation analysis
The results are presented as mean ± Standard Deviation (SD). Statistical analysis was performed using Graph Pad Prism, version 8 (GraphPad Software, La Jolla, CA, USA; www.graphpad.com). A difference was considered to be statistically significant at *P<0.05 and **P<0.01 and Not Significant (NS).
Reduction of HBV markers by thiourea derivatives and entecavir
As previously shown, thiourea derivatives were effective in reducing HBsAg and HBV DNA. Here, we checked again for the same and found that, in a cell culture model, thiourea derivatives effectively decreased HBV DNA compared to untreated (control) cells (Figure 1A). Thiourea derivatives have the potential to suppress the expression of HBV RNAs and it was found that they significantly reduced the expression of pre-genomic RNA as well as other HBV transcripts as compared to control and entecavir, which is currently used for the treatment of CHB infection (Figure 1B).
Followed by evaluating the secretion of HBV antigens and finding that thiourea derivatives reduced the secretion of HBV antigens significantly as compared with the control and entecavir-treated groups (Figure 1C). It shows the levels of HBV DNA, transcripts, and antigens in the cells treated with DSA-00, DSA-02, and ETV compared to untreated cells (Control). All experiments were performed three times and in triplicate. The statistical significance of the results was determined using the student's t-test. The asterisks (*) indicate the level of significance: *, p < 0.05; **, p < 0.001, indicating the results were statistically significant. They were better drugs because they reduced the expression and secretion of HBV RNAs and antigens significantly.
Alteration in protein profile following treatment with antiviral drug and thiourea derivatives
Thiourea derivatives and Entecavir both have distinct mode of action to reduced HBV infection. Therefore, speculated that to both drugs have different proteins targets in cells and how they alter the proteomic signature of cells. To investigate the alterations in protein profile during antiviral therapy in CHB infection, HepG2.2.15 cells were treated with standard antiviral drug Entecavir (ETV), which is a standard drug for treating CHB patients [26]. A wide-scale comparison of the proteome ETV-treated and untreated cells showed complete separation of the samples based on their similarity (Figure 2B). These was significant changes in the proteome of the ETV-treated group compared to the untreated group, with 607 proteins (274 UP and 246 Down-regulated) found to be significant (Figures 2C and 2D). According to Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis [27] the upregulated proteins were primarily linked to the ribosome, glycolysis, HIF-1 signaling, pyruvate metabolism, phagosome, pentose phosphate, necroptosis, tight junction, ferroptosis, peroxisome, fatty acid degradation, IL-17 signaling, mitophagy and pathways related to the cell cycle (p<0.05) (Figure 2E). On the other hand, the downregulated proteins were mostly linked to mRNA surveillance, Tricarboxylic Acid Cycle (TCA) cycle, protein processing in the ER, oxidative phosphorylation, insulin signaling, glucagon signaling and RNA transport (p<0.05) (Figure 2F). Besides, a large proportion of the proteins that were significantly altered were host antiviral proteins, whereas the proteins involved in bioenergetics were down regulated. These findings suggest that novel approaches are needed to restore the bioenergetics function and modulate the host Ribonucleic Acid Interference (RNAi) mediated antiviral defense system.
Thiourea derivatives induce specific protein modules to alter biological pathways
Though ETV and thiourea derivatives belonged to different classes of antivirals with distinct modes of action for suppressing HBV replication. ETV is known to have minimal impact on cellular energetics [28] whereas, thiourea derivatives have shown potential in inhibiting HBV replication [23]. We used WPCNA approach [29] to identify proteins modules specific to DSA-00, DSA-02, DSA-09, ETV and untreated cells for correlation patterns among proteins that may all work together in persistence of HBV infection (Figure 3A). WPCNA revealed 10 clusters (modules), corresponding to a total of 636 strongly linked proteins in DSA-00, DSA-02, DSA-09, ETV and untreated groups (Figure 3B). We got interested in modules whose expression pattern was similar to ETV and had some unique modules. DSA-00 has a similarity of approximately 70 percent with ETV (Black, Magenta, Red, Pink, Green and Yellow) and also has unique module (Grey), but DSA-09 has two common modules with ETV and DSA-00 (Black and Magenta). The ETV, DSA-00 and DSA-09 shared modules with proteins involved in Ribosome, proteasome, fatty acid biosynthesis, PPAR signaling and RNA transport pathways (Figure 3C). DSA-02 showed unique Brown module proteins that were associated with biological pathways such as glucagon signaling, tight junction, PPAR signaling, fatty acid degradation and spliceosome (Figure 3D). DSA-09 induced modules were common with DSA-00 and ETV modules. DSA-00 has a specific grey module other than common modules with ETV, DSA-02 and DSA-09. The grey module proteins were involved in protein processing in ER, oxidative phosphorylation, apoptosis, mitochondria biogenesis and protein folding pathways (Figure 3E). Additionally, the untreated group has unique modules (Blue and Turquoise) and the proteins in these modules were linked with regulation of actin cytoskeleton, tight junction, Hypoxia-Inducible Factor 1 (HIF-1) signaling, glycolysis/gluconeogenesis and protein processing in ER pathways (Figure 3F). Based on these findings we conclude that, proteomic changes induced by DSA-00 are 70% similar to that seen under the treatment with ETV (modules associated pathways in supplementary file 4). In addition, our results highlight that thiourea derivatives modulate unique protein modules majorly linked to mitochondrial and ER activity suggesting that the thiourea derivatives not on provide anti-viral support but also rejuvenates the mitochondria and hepatocyte health in CHB.
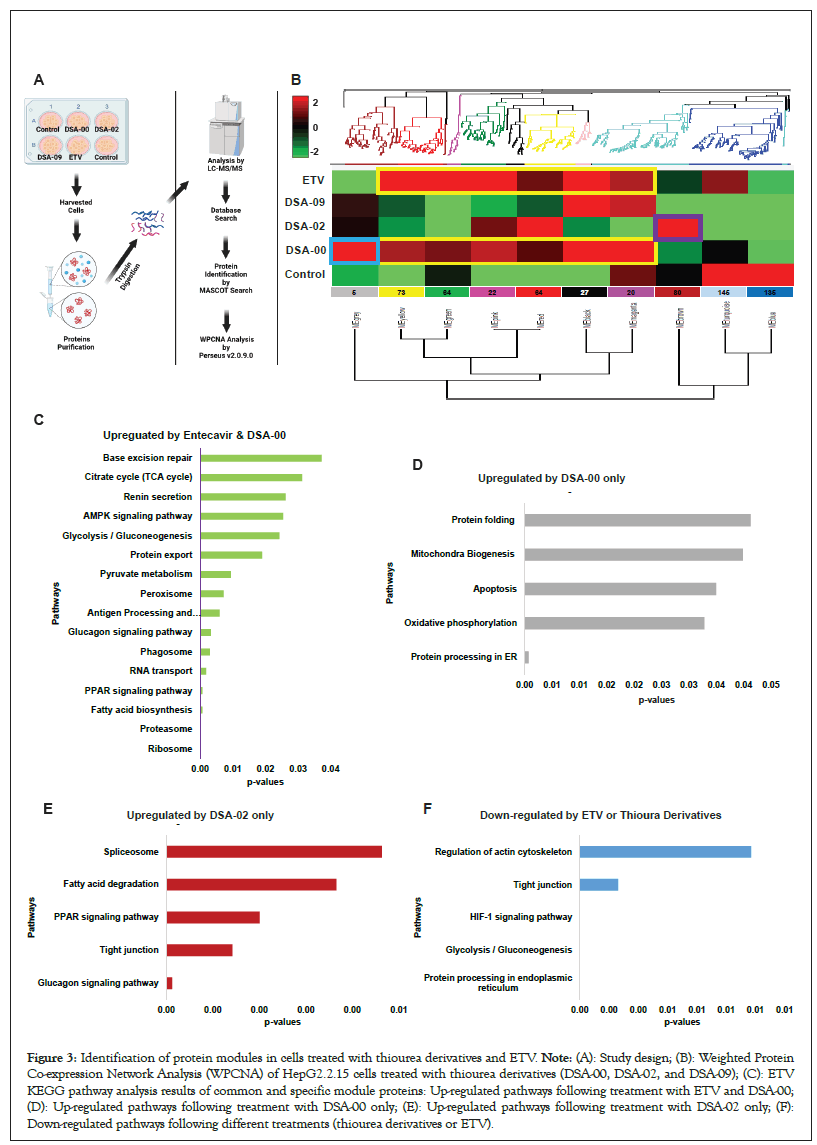
Figure 3: Identification of protein modules in cells treated with thiourea derivatives and ETV. Note: (A): Study design; (B): Weighted Protein Co-expression Network Analysis (WPCNA) of HepG2.2.15 cells treated with thiourea derivatives (DSA-00, DSA-02, and DSA-09); (C): ETV KEGG pathway analysis results of common and specific module proteins: Up-regulated pathways following treatment with ETV and DSA-00; (D): Up-regulated pathways following treatment with DSA-00 only; (E): Up-regulated pathways following treatment with DSA-02 only; (F): Down-regulated pathways following different treatments (thiourea derivatives or ETV).
Thiourea derivatives restore bioenergetics of exhausted hepatocytes
It is well established that HBx causes mitochondrial dysfunction and inflammation/injury in hepatocytes in CHB infection by disrupting mitochondrial respiration and membrane potential [30]. Thiourea derivatives, on the other hands, have been shown antioxidant activity [31] as well as bind to HBx and block the HBV-induced functions. Based on these findings, we hypothesized that thiourea derivatives may have the potential to restore mitochondrial dysfunction in HBV-related liver diseases. To investigate this hypothesis, HepG2.2.15 cells were treated with DSA-00, DSA-02, DSA-09 and ETV. The proteome profile was subjected to WPCNA that led to the identification of several modules of proteins that were differentially expressed between the groups. Pathway analysis of these modules showed that 31 in DSA-00 and 8 proteins in DSA-02 were linked with the antiviral response (Figure 4A). The brown module of DSA-02 contained eight proteins that were associated with enhanced host immunity and responsiveness to chronic infection (Figure 4B), whereas DSA-00 had twenty proteins providing negative regulation and ten associated with positive regulation to the HBV infection (Figure 4C). Furthermore, we observed that DSA-00 induced a unique grey module that contained only five proteins associated with mitochondrial functions like oxidative phosphorylation, membrane potential and apoptosis (Figure 4D). All antiviral response proteins that were up-regulated by DSA-00 (31 proteins), DSA-02 (8 proteins) and DSA-00 (5 proteins) were analyzed for their biological processes using the Protein Analysis through Evolutionary Relationships (PANTHER) classification system (v.14.0). This analysis revealed that nearly 50% of the proteins belonged to metabolic and cellular processes, suggesting that thiourea derivatives can restore bioenergetics in exhausted hepatocytes by targeting dysfunctional mitochondria (Figure 4E).
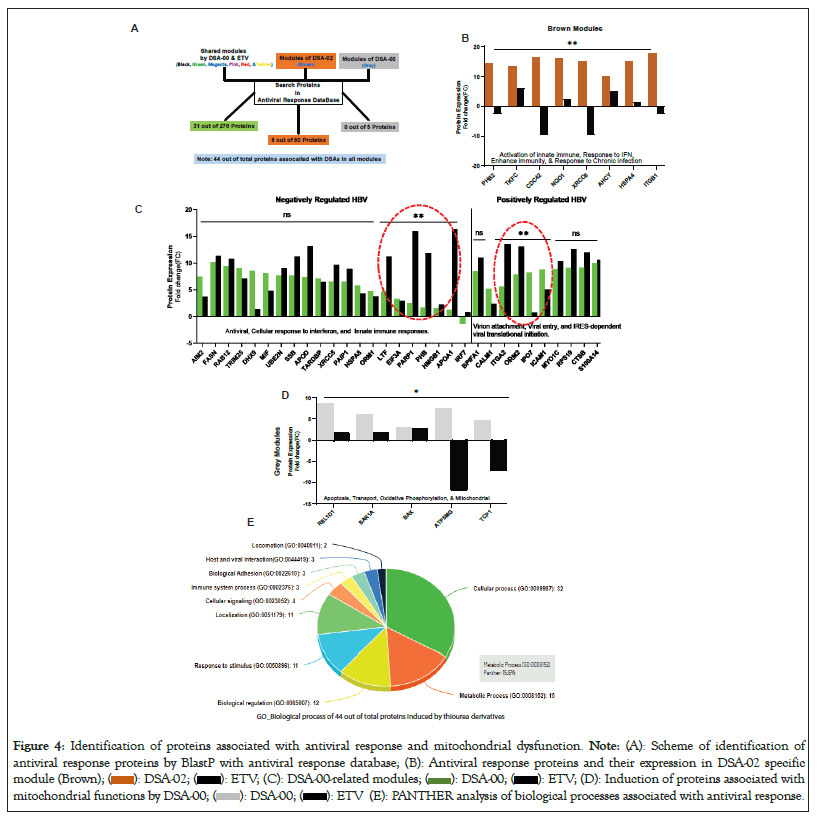
Figure 4: Identification of proteins associated with antiviral response and mitochondrial dysfunction. Note: (A): Scheme of identification of
antiviral response proteins by BlastP with antiviral response database; (B): Antiviral response proteins and their expression in DSA-02 specific
module (Brown);  Induction of proteins associated with
mitochondrial functions by DSA-00;
Induction of proteins associated with
mitochondrial functions by DSA-00;  (E): PANTHER analysis of biological processes associated with antiviral response.
(E): PANTHER analysis of biological processes associated with antiviral response.
Thiourea derivatives restore mitochondrial functions
After analyzing the metabolic and cellular process associated proteins, we found that most of the proteins were linked to the mitochondria (Figure 5A). Based on these results, we hypothesized that thiourea derivatives may have a role in mitochondrial-related functions. To validate these findings, we further blasted the proteins with the mitoproteome database [32] and found nine proteins (BAX, LMNA, HSPA5, HSPA4, PHB2, CTSB, MYO1C, PHB and FASN) were directly linked to mitochondria and its functions (Figures 5B and 5C). These results suggested that thiourea derivatives may improving mitochondrial health and functionality. To validate our hypothesis, HepG2.2.15 cells were treated with thiourea derivatives. Vitamin C was used as a positive control for anti-oxidative properties whereas ETV was used as known inhibitor for HBV infection. FACS analysis strategy revealed that thiourea derivatives had the potential to increase mitochondrial mass and restore the membrane potential of mitochondria (Figures 5D-5K). Therefore, the ratio of mass and membrane potential of mitochondria represents the functionality of the mitochondrial pool. Moreover, these thiourea derivatives were able to restore and maintain the pool of functional mitochondria in chronic HBV infected hepatocytes (Figure 5L). It shows restoration of mitochondria health in the cells treated with DSA-00, DSA-02, and DSA-09 compared to ETV, Vitamin C, and control cells. These observations clearly outline unique role of the thiourea derivative improving mitochondrial health not described earlier. Thus, the use of thiourea derivative in CHB care is could be complementary to the currently used antivirals.
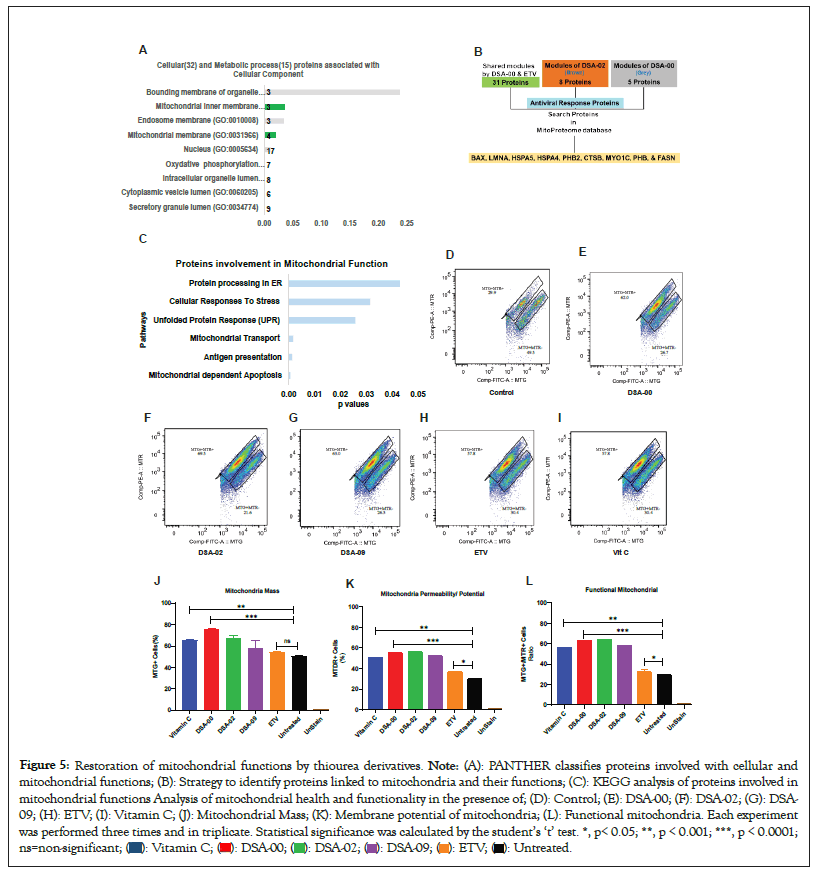
Figure 5: Restoration of mitochondrial functions by thiourea derivatives. Note: (A): PANTHER classifies proteins involved with cellular and
mitochondrial functions; (B): Strategy to identify proteins linked to mitochondria and their functions; (C): KEGG analysis of proteins involved in
mitochondrial functions Analysis of mitochondrial health and functionality in the presence of; (D): Control; (E): DSA-00; (F): DSA-02; (G): DSA-
09; (H): ETV; (I): Vitamin C; (J): Mitochondrial Mass; (K): Membrane potential of mitochondria; (L): Functional mitochondria. Each experiment
was performed three times and in triplicate. Statistical significance was calculated by the student’s ‘t’ test. *, p< 0.05; **, p < 0.001; ***, p < 0.0001;
ns=non-significant; 
CHB is a major risk to the progression to HCC, the sustained presence of the virus causes liver inflammation and exhaustion of hepatocytes [7]. Studies have shown that the replication of HBV and the expression of the non-structural protein HBx cause mitochondrial dysfunction and ER stress, such as changes in the unfolded protein response, biogenesis, mitophagy and the production of Reactive Oxygen Species (ROS) like superoxide and per-oxynitrite [7,33]. HBx has been shown to have diverse roles in regulatory HBV replication, cellular transcription and signal transduction pathways, proteasome activity and cell cycle progression and has also been linked to the progression of HBV-associated HCC [34]. Also, some cytosolic HBx may be found in the mitochondria of hepatocytes, where it can change the membrane potential of mitochondria and the amount of calcium in the cytosol through the mitochondrial Permeability Transition Pore (mPTP) [16,35]. The current study has focused on HBx as a potential therapeutic target to block HBV replication, restore mitochondrial function and reduce liver inflammation in CHB patients. One promising candidate for this approach is compound DSA-00 and its derivatives, which have been found to regulate HBx-induced activities, including HBV replication, HBx-induced kinase activity and HBx-modulated host RNAi machinery [23].Now, using a proteomic approach, we have found the proteins that have antiviral effects on HBV infection, improve mitochondrial and ER function and cause hepatocytes to become exhausted when CHB is present. To achieve this, the proteome profile of cell culture-based model of chronically infected hepatocytes was compared to treat with commonly used anti-viral ETV or different thiourea derivatives. Instead of performing conventional differential expression analysis based on predetermined protein sets or pathways, we conducted a modular analysis that used an unsupervised hierarchical clustering method to examine the link between groups of co-expressed proteins. We used the WPCNA method, which enables the identification of protein co-expression and determines how well a particular protein is connected to other protein pathways that are jointly involved in a biological process. The protein co-expression networks of intricate biological processes are segmented into modules by WPCNA, which identified 10 different modules, with black, magenta, red, pink, green and yellow modules most shared modules between DSA-00 and ETV treatment. According to enrichment analysis, these modules were mostly correlated with ribosome, proteasome, fatty acid biosynthesis, Peroxisome Proliferator-Activated Receptors (PPAR) signaling, RNA transport pathways. The brown module was unique for the DSA-02 treatment, with proteins significantly associated with biological processes such as glucagon signaling, tight junction, PPAR signaling, fatty acid degradation, spliceosome, etc. The grey modules had only five proteins, which were upregulated by the treatment with DSA-00. When these proteins were looked at by KEGG, it was found that they played a big role in mitochondrial biogenesis, oxidative phosphorylation and unfolded protein responses. Furthermore, we looked at proteins involved in the antiviral response and found 44 proteins in black, magenta, red, pink, green, yellow, brown and grey modules. Analysis of the correlation between the changed biological functions in DSA-00, DSA-02 and ETV groups and found that antiviral response proteins were associated with positively regulated and negatively regulated HBV infection in DSA-00+ETV groups. DSA-02 induced proteins that supported the antiviral response. Additionally, DSA-00 had a unique grey module, which had five proteins associated with mitochondrial functions. The aim of our study was to repurpose drugs that have the potential to reduce HBV infection and restore the exhausted hepatocytes that are notably exhausted during CHB infection. Restoration of dysfunctional mitochondria has been correlated with the improvement of cell health in the past, although not being previously linked to thiourea derivatives efficacy. Moreover, the blast hits with mitoproteome database [32] revealed that thiourea derivatives are suitable candidates that have the efficacy to restore dysfunctional mitochondria as well as have the potential to reduce HBV infection. Our study provides valuable insights into the potential of thiourea derivatives for the treatment of CHB and restoration of exhausted hepatocytes. These findings were further validated by the FACS, which found that treated cells with thiourea derivatives showed healthy, increased biogenesis and restored mitochondrial potential. Limitations of the investigation include the fact that it is a pilot study and that just one type of in-vitro CHB model (HepG2.2.15) was employed. Instead of measuring mitochondrial respiration, Oxygen Consumption Rate (OCR) and ATP production in the validation research, just test the mitochondrial health using flow cytometry.
Our research concentrated on CHB, which causes mitochondrial dysfunction, HBV reactivation and HBV replication. Thiourea derivatives known for the antiviral activity and restoration of host RNA interference mediated defense machinery. Here, we repurposed thiourea derivatives, they have the potential to restoration of the dysfunctional mitochondria. Mitochondrion-targeted antioxidants considerably enhanced mitochondrial (ROS) in cell exhaustion. Our study provides valuable insights into the potential of thiourea derivatives for the treatment of CHB and restoration of exhausted hepatocytes. Further experimental validation of our findings is necessary to confirm their potential clinical application.
The author would like to express their gratitude to PT, RPS and AKS for their valuable contributions to the study. The support and funding provided by VK and DS are greatly appreciated. Special thanks to JSM for their support expertise in proteomics analysis and sample preparation.
Conceptualization, J.K., PT, and J.S.M.; methodology, J.K., and J.S.M.; software, J.K. and RPS; validation, J.K., R.P.S. and P.T.; formal analysis, J.K.; investigation, J.K..; resources, J.K., and AKS; data curation, J.K.; writing-original draft preparation, J.K.,; writing-review and editing, J.S.M., and V.K.; visualization, J.K.; supervision, V.K.; project administration, DS; funding acquisition, V.K. All authors have read and agreed to the published version of the manuscript.
This work was supported by grants to VK from the Department of Biotechnology (DBT), Government of India (Grant No. BT/PR30082/Med/29/1341/2018) and J.C. Bose National Fellowship (Grant No. SR/S2/JCB-80)/2012) of the Department of Science and Technology, Government of India.
[Crossref] [GoogleScholar] [PubMed]
[Crossref] [GoogleScholar] [PubMed]
[Crossref] [GoogleScholar] [PubMed]
[Crossref] [GoogleScholar] [PubMed]
[Crossref] [GoogleScholar] [PubMed]
[Crossref] [GoogleScholar] [PubMed]
[Crossref] [GoogleScholar] [PubMed]
[Crossref] [GoogleScholar] [PubMed]
[Crossref] [GoogleScholar] [PubMed]
[GoogleScholar] [PubMed]
[Crossref] [GoogleScholar] [PubMed]
[Crossref] [GoogleScholar] [PubMed]
[Crossref] [GoogleScholar] [PubMed]
[Crossref] [GoogleScholar] [PubMed]
[Crossref] [GoogleScholar] [PubMed]
[Crossref] [GoogleScholar] [PubMed]
[Crossref] [GoogleScholar] [PubMed]
[Crossref] [GoogleScholar] [PubMed]
[Crossref] [GoogleScholar] [PubMed]
[Crossref] [GoogleScholar] [PubMed]
[Crossref] [GoogleScholar] [PubMed]
[Crossref] [GoogleScholar] [PubMed]
[Crossref] [GoogleScholar] [PubMed]
[Crossref] [GoogleScholar] [PubMed]
[Crossref] [GoogleScholar] [PubMed]
[Crossref] [GoogleScholar] [PubMed]
[Crossref] [GoogleScholar] [PubMed]
[Crossref] [GoogleScholar] [PubMed]
[Crossref] [GoogleScholar] [PubMed]
[Crossref] [GoogleScholar] [PubMed]
Citation: Kumar J, Tyagi P, Singh RP, Saini PK, Sharma D, Maras JS, et al. (2023) Thiourea Derivatives Restore Dysfunctional Mitochondria in Chronic Hepatitis B Infection. J Proteomics Bioinform.16:652.
Received: 25-Aug-2023, Manuscript No. JPB-23-26284; Editor assigned: 28-Aug-2023, Pre QC No. JPB-23-26284 (PQ); Reviewed: 11-Sep-2023, QC No. JPB-23-26284; Revised: 18-Sep-2023, Manuscript No. JPB-23-26284 (R); Published: 25-Sep-2023 , DOI: 10.35248/0974-276X.23.16.652
Copyright: © 2023 Kumar J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.