
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Mini Review - (2023)Volume 13, Issue 4
Sodium Lauryl Sulphate (SLS), an anionic detergent, is created through a chemical process. SLS was utilized in a variety of consumer and medical items, including toothpaste, mouthwash, shampoo, soap, face wash, and other cosmetics. Much research demonstrates that SLS has negative impacts. Many studies also suggest that SLS-containing products cause eye irritation, skin damage, mouth cytotoxicity, and cell protein damage, as well as hair loss. SLS has been demonstrated in scientific tests to have negative effects when used in high concentrations and incubated for a long period. SLS also has an impact on microbiologically on body. This article is not suggested the SLS containing product use should be closed but it incubation time and concentration is must be less. Many animal based research is describe for mouth wash containing product used 1%-2% w/w concentration, for skin care product (1% w/v), for shampoo (10%-25% w/v) concentration is used. Only 2.4% of SLS-containing products are licensed in the UK.
Shampoo, toothpaste, mouthwash, and other cosmetic and healthcare items are still in use today. For the creation of products in the current period, modern businesses use various chemicals, some of which can have toxic or cancerous effects. Sodium lauryl sulfate is included in a variety of medical and healthcare items. As an emulsifying cleaning agent, Sodium Lauryl Sulfate (SLS) is a common act as anionic surfactant. Numerous causal and modal-based investigations have shown that sodium lauryl sulfate is harmful. SLS is used in numerous products, including shampoo, toothpaste, mouthwash, soap, and cosmetics; however, it only be used once or twice a week is given lower effect [1]. Authorized medicinal items contain 0.2% w/w cream and 25% w/v medicated products, with several products likely to have a low SLS concentration. Only 2.4% of SLScontaining products are licensed in the UK. However, dermal (skin and ocular) or inhalation exposure causes its overuse and excessive concentration [1]. The main SLS is used in oral goods (tablets and capsules), where it rarely causes adverse reactions; it cannot be utilized in injections or ophthalmic treatments. According to scientific research, it has negative consequences due to its surfactant qualities, disruption of cell membranes, and protein conformational alteration.
As a detergent, Sodium Lauryl Sulfate (SLS) is used. A combination of sodium alkyl sulfate is sodium lauryl sulfate. SLS is created by sulfating chlorosulfonic acid or sulfur trioxide with commercially available lauryl alcohol. Following this process, sodium hydroxide or sodium carbonate in water is used to neutralize the byproduct. By hydrolyzing coconut oil or palm kernel oil to release their fatty acids, followed by hydrogenation, lauryl alcohol is often produced. Commercial samples of SLS are frequently blended with other alkyl sulfates, with dodecyl sulfate serving as the primary constituent, due to the synthesis process [2].
SLS is commercially available in powder and pellet form. The salt is a 12-carbon chain connected to a sulphate group, which gives the substance the amphiphilic qualities of a detergent (Figure 1). SLS appears as white or cream to pale yellow c rystals, flakes, or powder with a smooth feel, a soapy, bitter taste, and a subtle fatty odour. The pH of the alkaline salt is 7.0-9.5 (for a 1% w/v aqueous solution); small amounts are sufficient to significantly raise the pH of semi-solid preparations, causing compound degradation, for example when diluents such as emulsifying ointment, which contains SLS, are used for compounding steroidal preparations, because of this, as well as its skin irritant qualities [3].
Toxicity and hazard
SLS is a moderately toxic material with acute toxic effects or chronic toxicity including carcinogenicity irritation to the skin, eyes, mucous membranes, upper respiratory tract, and stomach. The toxicity of SLS derives primarily from its surfactant properties, producing disruption of cell membranes, cytokine release, and conformational changes of proteins, and also damage bacteria which present different parts of the body and they are good. The SLS limited concentration is less chance to give toxic effect different concentration range is described in Table 1.
| Product | Concentration |
|---|---|
| Skin care product | 1% w/v |
| Toothpaste and mouthwash | 1%-2% w/w |
| wetting agents in solid oral dosage form | 0.2%-1.5% w/w |
| Releaser in pessaries and suppositories | 0.4%-1% w/w |
| Shampoos contain a foaming or lathering agent | 10%-25% w/v |
Table 1: Concentration ranges of SLS in different products.
Skin toxicity caused by SLS
The skin is the largest portion of our body. Skin is destroyed in a variety of ways, including solar radiation, harmful chemical substances, and so on. Skin also contains microorganisms, some of which protect against various infections [4]. Various cosmetics and soaps are still used today that are prepared with various ingredients. Sodium Lauryl Sulfate (SLS) was employed in those goods. According to the preceding discussion, SLS acts as a detergent, and many animal tests demonstrate that SLS has a negative impact. However, SLS used in low concentrations does not cause harm, but when used in higher concentrations and for a longer period, it causes harm. Because of its surfactant qualities, it harms corneocytes, causing swelling, denaturation of keratin structure by direct binding, the elevation of stratum corneum pH, and change of lipid synthesis in this layer as shown in Figures 1-4.
Figure 1: Chemical structure of sodium lauryl sulfate.
Figure 2: The image shows different irritiants caused by using Sodium Lauryl Sulfate (SLS) containg products.
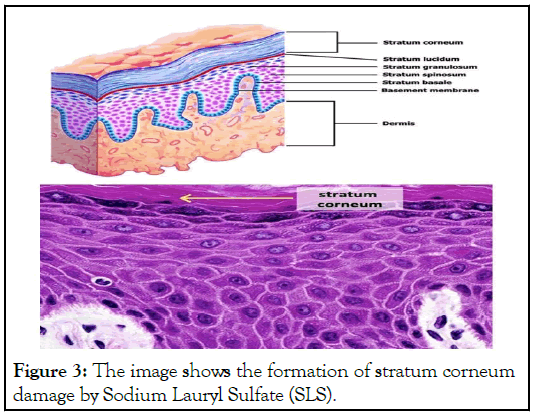
Figure 3: The image shows the formation of stratum spinosum due to Sodium Lauryl Sulfate (SLS) .
Figure 4: Trans Epidermal Water Loss (TWEL) caused by SLS.
According to Bondi, et al. [1], 0.01% to 50% concentration is recognized for cosmetics products and soap products. If a high concentration of SLS is used it may distort the cells protein. According to the study conducted by Prottly, et al. [5], after applying SLS on the third day causing scaling and cracking stratum corneum. According to the study by Lansdown, et al. [6], study conducted on animals, by applying 0.5 ml solution to a 2 cm2 patch of mouse skin, to know how much concentration and time required to know the harmful effects. They were also treated for 20 minutes, 60 minutes, and 120 minutes, which resulted in mild thickening and erythema after 1.5-2 hours of treatment, as well as 30%-40% squamous cells. After 2 hours, there was edema, leucocyte infiltration, and some engorgement of the more superficial blood vesicles.
Lansdown, et al. [6], investigated dilution by using 1% or 2.5% distilled water as well as 1% SLS on mice. After two days, there is little change, and after five days, the difference is more pronounced. Several investigations have revealed that 1% is less intense in the stratum spinosum. According to study of Olson [7], erythema was brought on by 1% and 5% solution.
Tsange, et al. [8], tested the application of aqueous cream BP on the forearm twice a day on six people. The results showed that those who were treated with SLS had a change in Stratum Corneum (SC) thickness, baseline TEWL, and rate of rise in TEWL during tape stripping there will be a 1.1 μm decrease in SC thickness.
Skin irritation studies on animal 0.1% concentration is describe as moderate effects. Mohammed, et al. [9], study on humans using 2 ml of aqueous cream BP for 10 minutes on an area of around 40 cm2 twice daily for 28 days led to increased protease activity and corneocytes size and maturity. According to their study, SLS is utilized in the UK for moisture purposes. The 0.9% SLS concentration decreases the thickness of healthy skin and also increases water loss permeability. Studies on animals show that a modest dose of SLS 0.1% causes a moderate effect due to its irritant qualities. The SC complex, which is primarily responsible for the human epidermal permeability barrier and is composed of corneocytes and intracellular lipids, forms a highly organized structure. At concentrations of 1% and less, the aqueous solution of SLS can irritate the skin and increase Tranepidermal Water Loss (TEWL).
SLS applied on teeth
The mouth is the most vital organ in the body, and it contains bacteria that protect against diseases and prevent them from entering other organs. Additionally, this microbe increases the mouth's enzyme activity. There are various types of toothpaste and mouthwash used to protect oral hygiene. Many types of toothpaste and mouthwash cause allergic reactions, according to Nowak, et al. [10], because SLS and fluoride are present in toothpaste and mouthwash. Based on the discussion above, SLD function as a detergents and fluoride also causes negative consequences. In-vitro research shows that mouthwash and toothpaste can harm oral musculature and gingiva. Fluoride ions are also found in toothpaste and mouthwash; they have both an antibacterial and an enzyme-stimulating effect. Additionally, it triggers oxidative stress and lipid peroxidation [9].
Lethal Dose LD50>5,000 mg/kg is regarded as non-toxic by the CPSF (Consumer Product Safety Commission). By using the Methyl Thiazolyl Tetrazolium (MTT) assay method, Shahidi,s et al. [11], examined the cytotoxicity of toothpaste and mouthwash. They also conducted investigations on how various toothpaste and mouthwash formulations affect oral bacteria. They prepared three plates with various concentrations and then examined them according to procedures lasting 15 and 30 minutes. In these plates, a positive control of 1 mg/ml and a negative control of 50 mg/ml are both displayed. Furthermore, Minimum Inhibitory Concentration (MIC) tests were conducted. Additionally, serial dilution was made, transferred, and incubated for 24 hours on MRS agar media. They tested mouthwashes with 1, 2, and 10 L volumes as well as kinds of toothpaste with concentrations of 1, 10, and 50 mg/mL. The cytotoxicity of the samples was above 90% at a concentration of 50 mg/L and a volume of 10 μl, while it was less than 1% at a concentration of 1 mg/mL and a volume of 1μl. All toothpaste and mouthwashes were positive for antibacterial action against S. mutants and L. acidophilus. The obtained MIC for all toothpastes and mouthwashes was between 0.0039 mg/mL and 0.0156 mg/mL, except for the corresponding values for sensodyne toothpaste and oral b mouthwash, which were 0.5 mg/mL and 0.0312 mg/mL when they were in contact with S. mutans and L. acidophilus for 1 min and 30 min, respectively [12].
A 0.8-1.1 g/kg is given modulatory effects based on lethal dose LD50 (Ingredient and formulation studies of items are called median lethal dose LD50 which helps to identify milligram substance per kilogram of our body weight) research [7]. Product Safety Labs (PSL), LD50 studies the leading cause of death is 6.0 g/kg [10]. After in gastric incubation with SLS diluted by diluted water 25% w/v, no adverse symptoms or microscopic activity are seen.
Ganpachi, et al. [12], investigated 16 kinds of toothpaste to determine cytotoxic effects and incubation time. They incubated epithelial cells and HeLa cells for 1 minute and found that different kinds of toothpaste damaged epithelial cells and HeLa cells at different times.
Acute eyes disease
Research on rabbits revealed that 10% SLS on animals when not washed caused opacity and stippling. According to published statistics, 28.2% and 30% of Sodium Lauryl Sulfate (SLS) used without washing and it showed mild to moderate irrational eyes. A concentration study on animals is carried out 20 or 30 seconds before the eyes are exposed. Eyes irrigate in 20 seconds, as evidenced by numerous studies. According to the study of Ghapanchi, et al. [12], 4 sec exposure to sodium lauryl sulfate elicited no opacities, but did dull corneal luster in four animals. The critical exposure time before damage is produced in the rabbit eye by this surfactant is between 4 and 10 sec.
Dermatological effect
SLS is mostly used in shampoo, although it is also found in toothpaste and hair care products. There is no strong scientific evidence that SLS-containing shampoo is carcinogenic, according to environmental research. People who want healthy hair should avoid using shampoos that include SLS. The majority of scientific data and most researchers classify it as irrational rather than carcinogenic [13,14].
Some people avoid it because they are allergic to chemicals and have sensitive hair and skin. Some persons with rosacea should avoid shampoo, according to the American Academy of Dermatology. SLS shampoo causes your hair to swell and additional layers of the cuticle to open, and it also causes hair color to fade. However, there is no substantial evidence that shampoo and SLS base products are carcinogenic. In cosmetics meant for prolonged skin contact, the concentration should not exceed 1% [15-18]
Mechanisms of action
SLS (Sodium Lauryl Sulphate) is a negatively charged surfactant formed by combining a saturated or mildly unsaturated hydrocarbon chain or a hydrophilic group with a strong acid such as sulphate (-O-SO3) or sulfonate (-SO3). This property causes SLS to operate as an irratry product. Because of its sarfance qualities, it disrupts the cell barrier and damages cell proteins by forming positively charged side groups, as well as impairing immunological response that are explained in Figures 2-4.
A repeated dose of SLS causes skin dryness and cracking, resulting in contact dermitis. Many studies have found that SLS causes basel transepidermal water loss (measured with an evaporimeter), skin thickness (measured with ultrasonic scanning), blood flow (detected with a laser Doppler flow metre) and skin colour (measured with a chroma metre).
Several studies also show that SLS has a direct effect on corneocytes, causing thickness swelling in size, denaturation of keratin structure via direct binding, and modulation of lipid formation in this layer as a result of pH shift [19]. Tranas Epithelial Water Loss (TEWL) also has an impact on SC shape. SLS is used to penetrate irritants and sensitive xenobiotics from the environment by removing lipids. According to Corck, et al. [20], some patients experience unfavorable effects when SLS is used on a regular basis, causing ssubstantial skin barrier breakdown, such as eczema. SLS concentrations of 0.1% to 2% commence free fatty acid elimination [21,22].
According to the evaluation of SLS toxicity studies, the SLS molecule is safe for toothpaste, mouthwash, soap, face wash, and various cosmetics. Much research has shown that SLScontaining products have a deleterious effect, but this varies based on concentration and time. Some products use varying amounts of SLS, while others dilute SLS, resulting in reduced or non-effect. When SLS-containing products are used for an extended period, the incubation period is also crucial. SLScontaining products can also harm the microbiology of our skin, mouth, and other parts of our bodies.
The authors declare no conflicts of interest
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Dhruv D (2023) The Study of Sodium Lauryl Sulfate (SLS) Toxicity. J Clin Toxicol. 13:542.
Received: 14-Jun-2023, Manuscript No. JCT-23-25096; Editor assigned: 16-Jun-2023, Pre QC No. JCT-23-25096 (PQ); Reviewed: 30-Jun-2023, QC No. JCT-23-25096; Revised: 07-Jul-2023, Manuscript No. JCT-23-25096 (R); Published: 14-Jul-2023 , DOI: 10.35248/2161-0495.23.13.542
Copyright: © 2023 Dhruv D. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.