Anatomy & Physiology: Current Research
Open Access
ISSN: 2161-0940
ISSN: 2161-0940
Review Article - (2022)
While telomerase maintains telomere length in dividing cells, a protective role has also been recently demonstrated in post-mitotic cells such as neurons where telomere length does change. Evidence accumulates for non-canonical functions of the telomerase protein TERT (Telomerase reverse transcriptase) in neurons that could have important implications for the treatment of neurodegenerative diseases. Various groups identified changes in neuronal gene expression and synaptic functions together with the activation of signalling pathways such as WNT as well as protein degradation pathways such as autophagy upon experimental increase of TERT levels in neurons and the brain of in vitro as well as in vivo models of neurodegenerative diseases such as Alzheimer’s Disease (AD) and Parkinson’s Disease (PD). The current short review summarises our knowledge on the roles of the telomerase protein TERT in the brain and as a possible therapeutic target of neurodegeneration.
Telomerase; TERT; Non-canonical; Brain; Neurodegenerative disease; Mouse model; Alzheimer’s disease; Parkinson’s disease
The best known and researched function of the enzyme telomerase is to maintain telomeres at the end of chromosomes. This role requires two minimal components: The catalytic subunit TERT (Telomerase Reverse transcriptase) and an inherent RNA component (TERC) which contains the template region for the de novo synthesis of telomeric hexanucleotide sequences (TTAGGG in mammals including humans). This addition compensates lost telomere sequences due to the End Replication Problem (ERP) during the normal semiconservative DNA replication process. In addition to this canonical function in telomere maintenance the TERT protein also has a number of non-canonical functions. These have been best investigated in relation to cancer where it plays an important role in tumourigenesis in addition to telomere synthesis that is a prerequisite for unlimited proliferation of these cells [1].
Some of these non-canonical functions are connected to a shuttling of the TERT protein from the nucleus, where it mainly fulfils its canonical role, but can also be involved in the regulation of gene expression and interaction with various signalling pathways, to other subcellular compartments including mitochondria [2-4]. This localization contributes to decreased oxidative stress, less sensitivity to apoptosis and thereby to a better survival of cells [3-5]. Recently, a role of mitochondrial TERT has also been described in vivo in human microvessels of patients with Coronary Artery Disease (CAD) as well as in mouse endothelial cells [6,7].
Activating telomerase and mechanisms of protection in models of neurodegeneration
Models of AD: The above described protective function of TERT in the brain subsequently prompted the use of different types of telomerase activators in neuronal and mouse models. Eitan and co-workers applied synthetic aryl compounds (AGS499) to a mouse model of ALS and found a delay of disease onset and a lower disease score. The same group employed this activator on a cellular AD model of cultured neurons and astrocytes that had been challenged with amyloid-β [13]. The authors found changes in genes responsible for brain plasticity as well as neuronal growth factors due to Ab treatment which could be reversed by the telomerase activator. A similar response was also achieved in a short-term treatment of an in vivo mouse model [13].
Recently, a new study from Ron dePinho’s lab demonstrated that somatic TERT activation in brain was able to counteract various aspects of AD such as amyloid pathology and synaptic dysfunction in various mouse models as well as in vitro in iPSCderived neurons [14].
For example the authors found in a TERT-haploinsufficient mouse model that several AD-related genes were changed in their expression including an upregulated APP (amyloid precursor protein) gene and the gene for the APP-processing enzyme Bace2. Reduction of TERT expression in this mouse model also suppressed various neuronal pathways such as neuronal differentiation, axon extension, transmission of neuronal pulses and regulation of action potential.
Similar to [13], the group also found a decrease of BDNF (brainderived neurotrophic factor) in this model. Importantly, in a triple transgenic (tg) mouse model of AD the authors found a decrease in TERT expression which was also identified in iPSCderived neurons from AD patients containing a duplication of the APP gene. These results are different to the findings of Spilsbury and co-authors who found no change in the amount of TERT protein in the hippocampus of spontaneous AD patients compared to healthy age-matched brains.
Possibly, the occurrence of genetic mutations in the models of [14], might play an exacerbating role here. The authors draw the conclusion that a decreased TERT expression together with changes in important AD- and brain-related signalling pathways might contribute to AD pathogenesis [14]. In order to increase TERT levels the group generated a tamoxifen-induced mouse model where TERT is specifically induced in neurons and crossed it with an AD mouse model. TERT induction resulted in a decrease of pathological amyloid together with an improved learning and cognition as well as less neuroinflammation in non-neuronal cells such as astrocytes and microglia.
These phenotypes were associated with changes in gene expression for synaptic signalling, plasticity and transmission as well as neuron projection and other functions. When TERT was activated in neurons derived from iPSCs of AD patients with an APP duplication the authors identified several members of the WNT pathway which had been described in association with TERT previously [17,18].
Various molecular methods also detected a direct binding of TERT protein with nuclear β-catenin, an important member of the WNT pathway. Importantly, confirming the non-canonical function of TERT within neurons, the authors obtained similar results on gene expression in the in vitro cell model when they used a catalytically inactive TERT variant confirming the noncanonical function for TERT which does not depend on enzymatic activity as soon in the Figure 1.
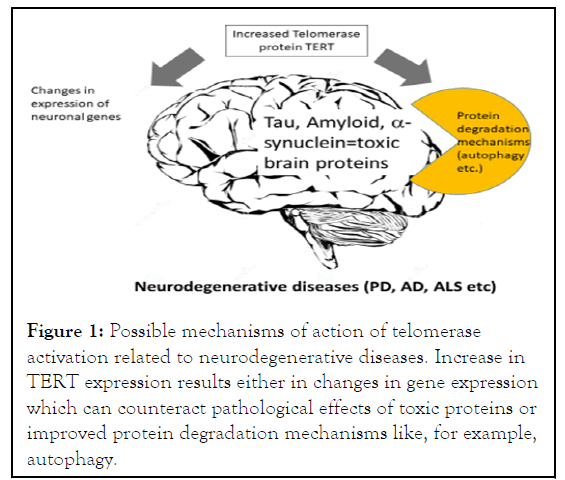
Figure 1: Possible mechanisms of action of telomerase activation related to neurodegenerative diseases. Increase in TERT expression results either in changes in gene expression which can counteract pathological effects of toxic proteins or improved protein degradation mechanisms like, for example, autophagy.
Together, these studies confirm that the telomerase protein TERT can counteract various pathological proteins in AD such as tau and β-amyloid by changing gene expression and decreasing oxidative stress in neurons. However, there are also reports of possible telomere-dependent functions of telomerase in other experimental models like late generation TERT knock-out mice characterised by shorter telomeres in the hippocampus and neocortex [17] where a re-introduction of telomerase/TERT using gene therapy with adenoviruses resulted in less DNA damage in the brain and improved cognitive functions of these mice. However, the authors did not show any re-elongation of short telomeres in the brain but only a decrease in general DNA damage and neuro-inflammation as well as improvement of neurogeneris and more tyrosine hydroxyalase (an enzyme involved in dopamine metabolism). Thus, while the authors hypothesise a telomeric function of TERT-re-expression in their TERT k.o. model, a non-canonical TERT functions cannot be excluded while other cell types such as astrocytes and neuronal stem cells could majorly contribute to the improved phenotype after TERT gene therapy.
Models of PD: Another group demonstrated an additional mechanism of protective TERT function against a different toxic brain protein: α-synuclein which is associated with Parkinson’s disease (PD). Using two different telomerase activators: A highly enriched plant extract TA-65 and a synthetic cycloastragenol (GRN510), Wan and co-authors (2021) used these compounds initially in a short-term treatment of 2 year old mice and neuronal cultures. Both models demonstrated an increase in TERT expression due to both activators. The results suggest that an increase in TERT expression targets neurons directly and also benefits the coordination and possibly cognitive functions in old individuals.
The authors progressed then to use these two activators on a mouse model of PD which was originally generated [19]. This model expresses human wild type α-synuclein, but the protein increased only at an age around 1 year [20], which corresponds well to the spontaneous disease being age-dependent. Wan found an increase in TERT expression in brains at 18 months when the analysis was performed [20].
Treating these mice from 4 months up to an age of 18 months with daily oral doses, the authors conducted various behavioural tests such as a rota-rod which analyses balance and coordination as well as a stride-length test which is equivalent to a gait test used to characterize human PD patients. The gait test showed a very convincing improvement of the stride length and a decrease of its variability with both telomerase activators in male and female mice [20]. In contrast, the rota-rod test showed a strong sex-dependency: While in females only the plant extract TA-65 improved speed, distance and time on the moving rod, in males only the synthetic GRN510 had a beneficial effect. This sexdifference could be explained by gender-differences between male and female brains which have been described previously for PD-related brain regions [21]. When the authors analysed brain pathology in 2 hippocampal regions (CA1 and CA2) and the neocortex they detected a significant decline in total, phosphorylated and aggregated α-synuclein in brains from mice that were treated with the two telomerase activators [15]. This result suggested a better protein degradation of α-synuclein which is causally associated with PD brain pathology. There is various protein degradation mechanisms involved in protein quality control known: Proteasomal degradation of mainly monomeric protein forms and different forms of autophagy (micro-, macro and Chaperon-Mediated Autophagy (CMA)). Macro-autophagy involves the formation of autophagosomes which degrade more complex protein forms (oligomeric and aggregated). Consequently, the authors analysed two components of this autophagy type: p62, an adapter protein and LC3B which is located on the membrane of autophagsomes. Both components decreased due to treatment with both telomerase activators significantly in hippocampal CA1 suggesting an activation of autophagy which seems to be responsible for a better degradation of α-synuclein. However, other protein degradation mechanisms might be also involved but have not been analysed. It is known that protein degradation mechanisms decline with age and this could explain why α-synuclein levels in the transgenic mouse model only increased after 1 year [20] which could be a reason for various spontaneous neurodegenerative diseases such as PD and AD only occurring at higher ages.
In summary, there is increasing interest in basic research as well as therapeutic approaches using increased telomerase levels in the brain to counter-act age related as well as neurodegenerationassociated functional decline of brain functions. So far, different methods such as the use of different telomerase activators or gene-therapeutic techniques have been employed to increase telomerase levels in neuronal cell models or the brain of mouse models. Different changes in gene expression of neurotrophic factors such as BDNF, synaptic signalling and plasticity or interaction with molecular pathways such as WNT and protein degradation pathways such as autophagy have been identified as possible underlying mechanisms and are summarised. While telomeres have been suggested to be involved in neurodegeneration, most authors emphasise non-canonical functions of the telomerase protein TERT to be responsible for the protective effects described. In summary, telomerase might be able to ameliorate the effects of pathological proteins associated with neurodegeneration such as tau, amyloid-β as well as α-synuclein which could be beneficial for the development of novel therapeutic strategies to combat age-related cognitive decline as well as aspects of neurodegenerative diseases.
[Cross ref] [Google scholar] [PubMed]
[Cross ref] [Google scholar] [PubMed]
[Cross ref] [Google scholar] [PubMed]
[Cross ref] [Google scholar] [PubMed]
[Cross ref] [Google scholar] [PubMed]
[Cross ref] [Google scholar] [PubMed]
[Cross ref] [Google scholar] [PubMed]
[Cross ref] [Google scholar] [PubMed]
[Cross ref] [Google scholar] [PubMed]
[Cross ref] [Google scholar] [PubMed]
[Cross ref] [Google scholar] [PubMed]
[Cross ref] [Google scholar] [PubMed]
[Cross ref] [Google scholar] [PubMed]
[Cross ref] [Google scholar] [PubMed]
[Cross ref] [Google scholar] [PubMed]
[Cross ref] [Google scholar] [PubMed]
[Cross ref] [Google scholar] [PubMed]
[Cross ref] [Google scholar] [PubMed]
[Cross ref] [Google scholar] [PubMed]
[Cross ref] [Google scholar] [PubMed]
Citation: Saretzki G (2022) The Role of Telomerase in Brain and Neurodegenerative Diseases. Anat Physiol. S8:381.
Received: 19-Apr-2022, Manuscript No. APCR-22-17282; Editor assigned: 22-Apr-2022, Pre QC No. APCR-22-17282 (PQ); Reviewed: 06-May-2022, QC No. APCR-22-17282; Revised: 13-May-2022, Manuscript No. APCR-22-17282(R); Published: 20-May-2022 , DOI: 10.35248/2161-0940.22.S8.381
Copyright: © 2022 Saretzki G. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.