Internal Medicine
Open Access
ISSN: 2165-8048
ISSN: 2165-8048
Research Article - (2022)Volume 12, Issue 2
Silent Information Regulator Proteins (SIRT) plays key roles in glucose and lipid metabolism and homeostasis. The SIRT1 inhibitor was used to investigate the mechanism of the SIRT1/FoxO1 (Forkhead box O1) pathway in mice and its function in the prevention of insulin resistance in skeletal muscle. Forty male mice were randomly divided into a control group and three experimental groups (NH: High-fat diet, HE: High-fat diet plus exercise, and HES: High-fat diet plus exercise and SIRT1 inhibitor). After 14 weeks, the fat weight, blood glucose level, and HOMA-IR score in HES were significantly increased compared to those in HE (P<0.01). The same parameters were significantly decreased in HE compared to those in the NH group (P<0.01). Mitochondrial 8-OHdG (8-hydroxy-2 deoxyguanosine) level of the HES group was significantly higher than that of the HE group (P<0.01). Compared with NH, the 8-OHdG and 4-HNE (4-hydroxynonenal) level were significantly decreased in HE (P<0.01). Histologic evaluation demonstrated mitochondrial vacuoles in HES, while a clean arrangement of mitochondrial cristae was observed in HE. FoxO1 acetylation was enhanced in HES compared to that in HE (P<0.01). The insulin resistance was induced in mice after 14-week high-fat diet, while glucose metabolism was significantly improved by aerobic exercise. The physiologic adaptations induced by exercise were not observed in the presence of an SIRT1 inhibitor. Thus, the SIRT1/FoxO1 pathway effectively regulates glucose metabolism during aerobic exercise and also prevents fat-induced peroxidation injury in skeletal muscles.
Silent Information Regulator Proteins 1 (SIRT1); Forkhead box O1 (FoxO1); Skeletal muscle; Aerobic exercise; Glucose homeostasis.
NC: Control group; NH: High-fat diet group; HE: High-fat diet plus exercise group; HES: High-fat diet plus exercise and SIRT1: Inhibitor group; FBG: Fasting Blood-Glucose; HOMA-IR: Homeostasis Model Assessment Insulin Resistance. Mn-SOD: Mn Superoxide Dismutase; CAT: Catalase; NAD+: Nicotinamide Adenine Dinucleotide; NADH: Reduced Nicotinamide Adenine Dinucleotide; 8-OHdG: 8-hydroxy-2'-Deoxyguanosine; 4- HNE:4-Hydroxynonenal; OGTT: Oral Glucose Tolerance Test; GLUT4: Glucose Transporter 4; PGC-1α: Peroxlsome Proliferator-activated Receptor-γ Coactlvator-1α; SIRT1: Sirtuin-1; FoxO1: Deacetylation of Forkhead box protein 1; GPX4: Glutathione Peroxidase 4; NOX1: Nicotinamide Adenine Dinucleotide Phosphate Oxidase 1; GAPDH: Glyceraldehyde-3-Phosphate Dehydrogenase.
Silent Information Regulator Proteins (SIRT) plays diverse and extensive roles in glucose and lipid metabolism. Seven types of mammalian sirtuins (SIRT1-SIRT7) have been identified [1]. Sirtuins are capable of regulating cellular mechanisms, including mitochondrial energy homeostasis, antioxidant activity, glucose and lipid metabolism, and DNA repair. These proteins can also regulate transcription, cell differentiation, and genome stability. The beneficial effects of sirtuins have been confirmed across various species. SIRT1 has been studied in recent years for its protective effects on inflammation, Alzheimer's Disease (AD), vascular aging, heart disease, atherosclerosis, and diabetes [2-4]. SIRT1 is expressed in hepatic, muscular, cardiovascular, and pancreatic tissues, where it regulates glucose metabolism through the deacetylation of Forkhead box protein 1 (FoxO1) [1,5]. Glucose metabolism in skeletal muscles plays an important role in glucose homeostasis, which prevents insulin resistance and similar metabolic disturbances. The expression of sirtuins in skeletal muscles can be altered by exercise, which in turn regulates changes in mitochondrial biogenesis, aerobic metabolism, and cellular antioxidants [6]. Exercise can lead to beneficial physiologic adaptations and prevent metabolic disorders like Type 2 Diabetes Mellitus (T2DM). The mechanism of these physiologic adaptations is a research hotspot in the field of exercise and health [7]. Many studies have demonstrated that the insulin resistance was significantly improved after exercise, but the involvement of SIRT1 is not clearly understood [8]. In this study, SIRT1 inhibitor was used to assess the role of the SIRT1/FoxO1 pathway in skeletal muscle glucose metabolism during aerobic exercise with the aim to provide a basis for potential therapeutic involvement of SIRT1 in insulin regulation and prevention of T2DM.
Grouping of experimental animals
Forty 5-week SPF C57BL/6 J male mice (license number: SCXK (Guangdong) 2018-0002), weighing 18 ± 2 g and basic animal feed were procured from Guangdong Medical Experimental Animal Center. The mice were randomly divided to four groups: a Normal Control group (NC), a high-fat diet group (NH), a High-fat diet plus Exercise group (HE), and a high-fat diet plus exercise and SIRT1 inhibitor group (HES). The NC group received daily basic feed (AIN-93G) without any exercise. The HE and HES groups received the Specific Pathogen-Free (SPF) grade high-fat diet, which consisted of 60% Kcal and was provided by Nantong Trophy. There was no significant difference in body weight among the four groups (NC: 19.72 ± 1.94 g, NH: 19.25 ± 2.37 g, HE: 19.24 ± 2.28 g, HES: 19.67 ± 1.86 g, P>0.05). Selisistat (EX 527) was used as the SIRT1 inhibitor and was injected intraperitoneally at a dose of 10 mg/kg (soluble in DMSO) [9]. All animal experiments were approved by the Scientific Research Ethics Sub-committee, School of Physical Education, and South China Normal University (Guangzhou, China; approval number: SCNU-SPT-2019-002).
Exercise scheme
During the adaptation period, the mice ran on the treadmill for 10 min each day at a speed ranging from 10 to 13 m/min. The formal exercise training period consisted of 14 weeks with moderate intensity (approximately 75% maximum oxygen uptake) treadmill exercise intervention. The slope of the treadmill was 0 degree during the entire training period. The initial speed of the treadmill was 14 m/min. The speed of the treadmill was increased to 15 m/min for the third and fourth weeks. The speed was increased to 16 m/min for the fifth and sixth weeks and to 17 m/min for the seventh and eighth weeks. For the ninth week, the speed was increased to 18 m/min, and it was increased to 19 m/min for the eleventh week. The speed was then maintained at 19 m/min until the fourteenth week [10]. None of the animals had a history of treadmill exercise before the intervention.
Tissue analysis
We measured the lean body weight and fat content of mice 12 h after the last exercise session, using a nuclear magnetic resonance mouse body composition analyzer. Fasting for 12 h, the blood glucose level of the mice was measured and an Oral Glucose Tolerance Test (OGTT) was conducted. We intragastrically administered 25% glucose (2.5 g/kg body weight). Roche blood glucose meter was used to detect glucose levels in blood collected from tail tips at 0, 60, 90, and 120 min. To collect blood and tissue samples, the mice were anesthetized using a 10% chloral hydrate solution. Blood samples of mice were collected from the right eye and placed at 24°C-26°C for 30 min. The samples were centrifuged at a speed of 845 × g for 10 min at 4°C. The serum was packed separately. The lateral head of the gastrocnemius was removed and washed with 0.9% NaCl. The sample was frozen in liquid nitrogen and refrigerated at -80℃. Gastrocnemius mitochondria were isolated by differential centrifugation. The right gastrocnemius muscle was fixed with paraformaldehyde, and paraffin sections were prepared for staining with hematoxylin and eosin. A part of the lateral head of the gastrocnemius was fixed with 4% glutaraldehyde to prepare ultrathin electron microscope sections.
Reagents and instruments
A tissue mitochondrial separation kit was obtained from Beyotime (China). We purchased 8-Hydroxy-2 Deoxyguanosine (8-OHdG) of gastrocnemius mitochondria from Sangon (Shanghai) and 4-Hydroxynonenal (4-HNE) of gastrocnemius muscle from Nanjing Jiancheng Institute of Biological Engineering. Blood glucose levels were detected using Roche Products and Roche Diagnostics. The following rabbit antimouse antibodies were used: Glucose Transporter 4 (GLUT4) (ABclonal), SIRT1, Peroxisome proliferator-activated receptor Gamma Coactivator 1-alpha (PGC-1α), Glutathione Peroxidase 4 (GPX4), Nicotinamide Adenine Dinucleotide Phosphate Oxidase 1 (NOX1) (both from Santa Cruz), FoxO1 (Abcam), Acetyl-FoxO1 (Thermo Fisher Scientific). GAPDH was used as an internal reference (GAPDH anti-rabbit polyclonal antibody; Abcam). Mn-SOD, CAT, coenzyme I (NAD+/NADH), and glycogen levels in the gastrocnemius muscle were measured using kits purchased from Nanjing Jiancheng Institute of Biological Engineering.
In this study, we used a small animal nuclear magnetic resonance imaging instrument (Newmai, NM42-040H-1) along with a Synergy H4 multifunctional enzyme labeling instrument (Thermo Scientific). An electrophoresis and membrane transfer system (Bio-Rad), a PVDF membrane (Bio-Rad), and an electron microscope (Hitachi, HT 7700) were also used.
Insulin resistance index
Insulin, 8-OHdG, and 4-HNE levels were determined using Enzyme Linked Immunosorbent Assay (ELISA). The expression of gastrocnemius-related proteins was detected by western blotting. The dilution of GLUT4, Acetyl-FoxO1, and FoxO1 was 1:500, and the dilution of SIRT1 was 1:1000. The dilution of PGC-1α and GAPDH was 1:1500. NAD+/NADH, Mn-SOD, and muscle glycogen levels were measured using colorimetry.
Statistical analysis
All data were expressed as the mean standard deviation (xs ). The SPSS statistics package (SPSS Inc., Chicago, IL) was used to analyze the data by one way ANOVA, and the LSD method was used to make pairwise comparisons.
Body composition, blood sugar and glucose tolerance
The body weight, body fat content, Fasting Blood Glucose (FBG) level, and HOMA-IR score in the NH group increased significantly at the end of 14-week high-fat diet (P<0.01), while these parameters were significantly decreased in the HE group than in the NH group (P<0.01). The body weight, body fat content, FBG level, and HOMA-IR score in the HES group were significantly higher than in the NC and HE groups (P<0.05, P<0.01). The wet muscle weight and glycogen content in the NH group were reduced compared to those in the HE group (P<0.01) (Figure 1).
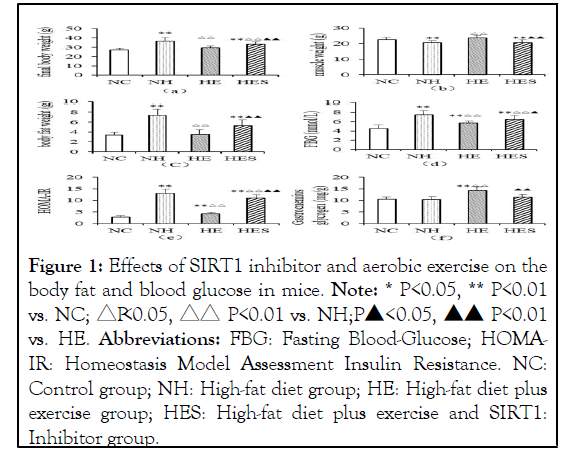
Figure 1: Effects of SIRT1 inhibitor and aerobic exercise on the body fat and blood glucose in mice. Note: * P<0.05, ** P<0.01 vs. NC; △P<0.05, △△ P<0.01 vs. NH;
Abbreviations: FBG: Fasting Blood-Glucose; HOMAIR: Homeostasis Model Assessment Insulin Resistance. NC: Control group; NH: High-fat diet group; HE: High-fat diet plus exercise group; HES: High-fat diet plus exercise and SIRT1: Inhibitor group.
As per OGTT results, glucose levels of the NH group reached a peak of 11.64 ± 0.59 mmol/L after 120 min, following initial administration. The glucose levels of the HES and HE groups 120 min following initial administration were 10.94 ± 0.43 mmol/L and 9.26 ± 0.72 mmol/L, respectively. The clearance rate of blood glucose in the HE group was significantly higher than that in the HES group (Figure 2).
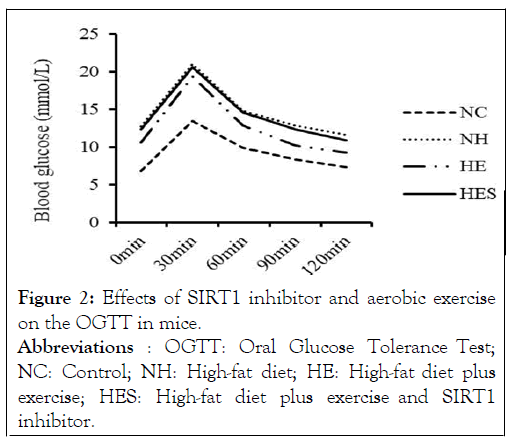
Figure 2: Effects of SIRT1 inhibitor and aerobic exercise on the OGTT in mice. Abbreviations : OGTT: Oral Glucose Tolerance Test; NC: Control; NH: High-fat diet; HE: High-fat diet plus exercise; HES: High-fat diet plus exercise and SIRT1 inhibitor.
Oxidative stress alterations
The activity of antioxidant enzymes Mn-SOD and CAT, blood glucose levels, and the ratio of NAD+/NADH in the NH group decreased significantly compared to that in the NC group (P<0.01), (P<0.01). The activity of antioxidant enzymes and NAD+/NADH levels in the HE group were significantly higher than in the HES group (P<0.01). The level of peroxide damage markers 8-OHdG and 4-HNE in the NH group was significantly higher than in the NC and HE groups (P<0.01); 8-OHdG and 4-HNE levels in the HES group were significantly increased in comparison to those in the HE group (P<0.01) (Figure 3).
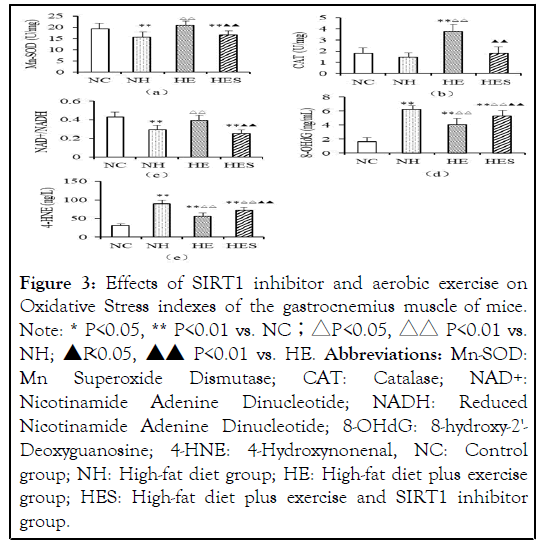
Figure 3: Effects of SIRT1 inhibitor and aerobic exercise on Oxidative Stress indexes of the gastrocnemius muscle of mice. Note: * P<0.05, ** P<0.01 vs. NC;△P <0.05, △△ P<0.01 vs. NH; 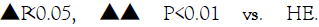
Abbreviations: Mn-SOD: Mn Superoxide Dismutase; CAT: Catalase; NAD+: Nicotinamide Adenine Dinucleotide; NADH: Reduced Nicotinamide Adenine Dinucleotide; 8-OHdG: 8-hydroxy-2'- Deoxyguanosine; 4-HNE: 4-Hydroxynonenal, NC: Control group; NH: High-fat diet group; HE: High-fat diet plus exercise group; HES: High-fat diet plus exercise and SIRT1 inhibitor group.
Histomorphology of skeletal muscle and mitochondria
There was an increase in the cross-sectional area and the number of skeletal muscle cells in tissue samples in the HE group compared to that in the HES group (Figure 4). The mitochondria from the NH group-mice were abundant in vacuoles, while the HE group-mice possessed mitochondria with neatly arranged cristae. The mitochondria of the HES group displayed vacuoles and ruptured cristae (Figure 5).
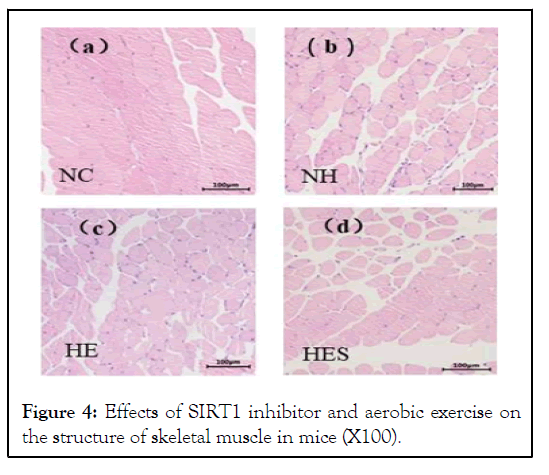
Figure 4: Effects of SIRT1 inhibitor and aerobic exercise on the structure of skeletal muscle in mice (X100).
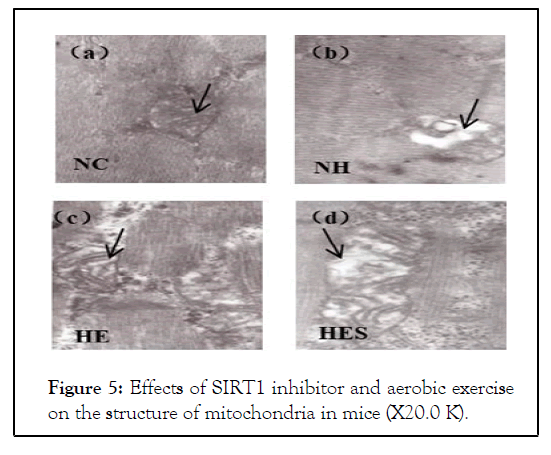
Figure 5: Effects of SIRT1 inhibitor and aerobic exercise on the structure of mitochondria in mice (X20.0 K).
The cross-sectional area of skeletal muscle of the HES group was lower than that of the HE group. Skeletal muscle cell space of the HES group was increased than that of the HE and NC groups. NC: Control group; NH: High-fat diet group; HE: High-fat diet plus exercise group; HES: High-fat diet plus exercise and SIRT1 inhibitor group.
The mitochondria from the NH group-mice were abundant in vacuoles, while the HE group-mice possessed mitochondria with neatly arranged cristae. The mitochondria of the HES group displayed vacuoles and ruptured cristae. NC: Control group; NH: High-fat diet group; HE: High-fat diet plus exercise group; HES: High-fat diet plus exercise and SIRT1 inhibitor group.
Expression of SIRT1/FoxO1 pathway components
The NH group had significantly decreased expression of GLUT4 (P<0.05, Figure 6a), PGC-1α (Figure 6b), SIRT1 (Figure 6c), and GPX4 (Figures 6d and 6e) (P<0.01) compared to those in the control group. FoxO1 acetylation and NOX1 (Figure 6f) were increased (P<0.01, P<0.05) in the NH group. The expressions of GLUT4, PGC-1α, GPX4 and SIRT1 in the HE group weresignificantly higher than those in the HES group (P<0.01, P<0.05). Expression of NOX1 in the HES group was significantly increased in comparison to that in the HE group (P<0.05).The expression of FoxO1 acetylation in the HES group was significantly higher than that in the other three groups (Figure 6d).
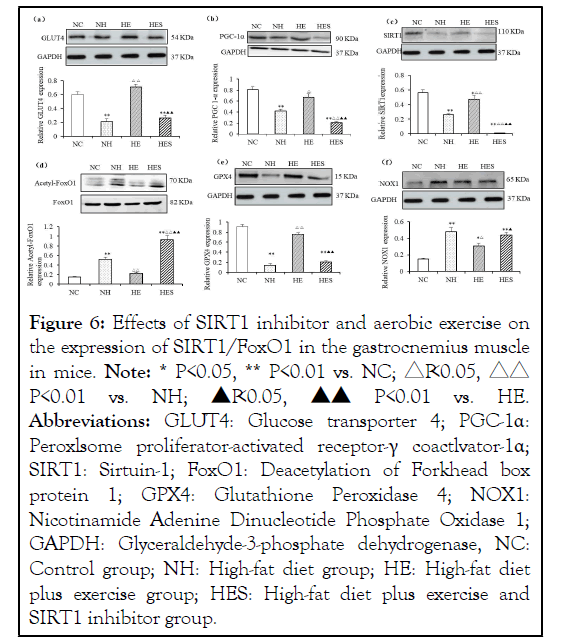
Figure 6: Effects of SIRT1 inhibitor and aerobic exercise on
the expression of SIRT1/FoxO1 in the gastrocnemius muscle
in mice. Note: * P<0.05, ** P<0.01 vs. NC; △P<0.05, △△
P<0.01 vs. NH;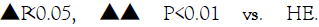 Abbreviations: GLUT4: Glucose transporter 4; PGC-1α:
Peroxlsome proliferator-activated receptor-γ coactlvator-1α;
SIRT1: Sirtuin-1; FoxO1: Deacetylation of Forkhead box
protein 1; GPX4: Glutathione Peroxidase 4; NOX1:
Nicotinamide Adenine Dinucleotide Phosphate Oxidase 1;
GAPDH: Glyceraldehyde-3-phosphate dehydrogenase, NC:
Control group; NH: High-fat diet group; HE: High-fat diet
plus exercise group; HES: High-fat diet plus exercise and
SIRT1 inhibitor group.
Abbreviations: GLUT4: Glucose transporter 4; PGC-1α:
Peroxlsome proliferator-activated receptor-γ coactlvator-1α;
SIRT1: Sirtuin-1; FoxO1: Deacetylation of Forkhead box
protein 1; GPX4: Glutathione Peroxidase 4; NOX1:
Nicotinamide Adenine Dinucleotide Phosphate Oxidase 1;
GAPDH: Glyceraldehyde-3-phosphate dehydrogenase, NC:
Control group; NH: High-fat diet group; HE: High-fat diet
plus exercise group; HES: High-fat diet plus exercise and
SIRT1 inhibitor group.
The expression of GLUT4 (a), PGC-1α (b), SIRT1 (c), GPX4 (e) and NOX1 (f) relative to GAPDH in the gastrocnemius muscle in mice. (d) The expression of acetyl-FoxO1 relative to FoxO1 in the gastrocnemius muscle in mice.
Effects of SIRT1 inhibitor and aerobic exercise on oxidative stress of gastrocnemius muscle in high-fat mice
A high-fat diet reduced the activity of antioxidant enzymes in the mouse gastrocnemius muscle and significantly aggravated mitochondrial peroxidation damage. Exercise can effectively improve the activity of antioxidant enzymes in muscles and can increase the ability of the body to resist oxidative damage. Therefore, reducing the concentration of 4- HNE and other oxidative damage byproducts has a protective effect on mitochondria. The concentration of 8-OHdG in the mitochondria of the exercise group was significantly lower than that of the SIRT1 inhibitor group. Exercise can promote the expression of SIRT1 in skeletal muscles and can increase mitochondrial biogenesis and oxidative metabolism [6]. The improvement in mitochondrial structure and function is helpful in maintaining insulin sensitivity and normal glucose and lipid metabolism in skeletal muscles. Aerobic exercise can significantly increase the expression of GLUT4 on skeletal muscle cell membranes and promote glucose transport into the muscle tissue. This could be an important reason behind why glycogen in the gastrocnemius muscle of the HE group increased significantly, and its blood glucose level was significantly lower than that in the NH group.
The HES group showed significant reversal of the physiologic changes brought about by exercise on insulin resistance. The activities of antioxidant enzymes such as Mn-SOD and CAT in the HES group were significantly lower than those in the HE group, but there was no significant difference between NH and HES groups. The expression of GPX4 was significantly decreased in HES, which can cause severe lipid peroxidation damage. The concentration of the mitochondrial peroxidation damage marker 8-OHdG was significantly higher in the HES group than in the HE group. The increased NOX1 in HES group promotes peroxide damage and apoptosis. A decrease in the expression of GLUT4 was observed in the gastrocnemius muscle of the HES group-mice in comparison to that of the control and HE groupmice, along with a decrease in glucose transport, which led to low muscle glycogen content and high blood glucose concentration. The PGC-1α concentration was decreased, and the structure and function of gastrocnemius mitochondria were damaged in the HES group. This further shows that SIRT1 plays a key role in maintaining mitochondrial homeostasis. Studies on nematodes and mice have shown that increasing the activity of NAD and SIRT1 can enhance the function of mitochondria, maintain metabolic functions, and prolong life span [1,16]. In this experiment, the NAD /NADH ratio of skeletal muscles increased after exercise, which further increased SIRT1 expression. This data is consistent with the results of previous studies [1]. GPX4 specifically catalyzes lipid peroxides to inactivate them, thereby protecting cells.
The role of SIRT1/FoxO1 pathway of skeletal muscle in preventing insulin resistance by aerobic exercise
The experimental mice were fed a high-fat diet, and they adhered to an exercise regimen and received an SIRT1 inhibitor. The results showed that Selisistat had a good inhibitory effect on SIRT1, and the expression of SIRT1 in the gastrocnemius decreased significantly. The acetylation of FoxO1, which is present downstream of SIRT1, was increased significantly. Acetylation and deacetylation play important roles in glucose metabolism. NAD+-dependent SIRT1 can promote the deacetylation of key metabolic enzymes, accelerate glucose decomposition, and play an important role in the prevention and treatment of insulin resistance [17,18]. The results showed that exercise could improve insulin resistance in mice by promoting NAD+ expression, activating SIRT1, and reducing FoxO1 acetylation. Exercise can promote the expression of PGC1-α in skeletal muscles, while SIRT1 inhibitors can reduce PGC1-α. Research has shown that SIRT1 may directly affect PGC1-α, and it may also act on PGC1-α through FoxO1.
Active substances such as resveratrol, apple polyphenol extract, ginsenoside, astaxanthin, and vitamin D can promote theexpression of SIRT1 and activate AMPK, FoxO1, PGC1-α, and Nrf2 downstream of SIRT1 [19-24].The activation of the SIRT1/FoxO1 pathway plays a role in the improvement of brain function and in the treatment of depression and Parkinson's Disease (PD). Serum SIRT1 levels in patients with PD and healthy controls were measured using ELISA, and resulting SIRT1 levels in patients with advanced PD decreased in relation to disease severity and cognitive dysfunction [25]. NAD+, miR-34a, and miR204 [22] are the key regulatory factors upstream of SIRT1. The influence of exercise on these regulatory factors in the prevention and treatment of metabolic diseases, depression, and PD needs to be studied further [26-28].
In conclusion, SIRT1 inhibitor significantly reversed the physiologic adaptations brought about through exercise in mice with insulin resistance induced by a high-fat diet. Addition of the SIRT1 inhibitor resulted in increased body fat content, higher FBG level, and oxidative damage along with the destruction of structural mitochondrial tissue. Glycogen stores depleted, acetylation of FoxO1 increased, and antioxidant enzymatic activity decreased in the SIRT1 inhibitor group. These results support the claim that exercise improves insulin resistance in the skeletal muscle of mice that are fed a high-fat diet through the deacetylation of FoxO1 by SIRT1. The SIRT1/FoxO1 regulatory pathway is crucial in improving insulin resistance and preventing peroxidation damage in skeletal muscles of mice during aerobic exercise.
We would like to thank Editage (www.editage.cn) for English language editing.
This work was funded by Talent Introduction Special Funds of Lingnan Normal University to Yuqian Liu (ZL2008) and Haitao Wang (ZL2009). This work was supported by Guangdong Provincial Key Laboratory of Development and Education for Special Needs Children to Yuqian Liu (TJ202103).
The authors have no relevant financial or non-financial interests to disclose.
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Haitao Wang, Yuqian Liu, Wenqian Yang and Guang Yang. The first draft of the manuscript was written by Haitao Wang and Yuqian Liu revised the manuscript. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
The datasets generated during and/or analysed during the current study are available in the appendix of the article and are only used for manuscript review. These datasets are the original experimental data, which are available from the corresponding author on reasonable request.
All animal experiments were approved by the Scientific Research Ethics Sub-committee, School of Physical Education, South China Normal University (Guangzhou, China; approval number: SCNU-SPT-2019-002).
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
Citation: Wang H, Yang W, Yang G, Liu Y (2022) The Regulatory Role of the SIRT1/Foxo1 Pathway in the Prevention of Insulin Resistance in Skeletal Muscle by Aerobic Exercise in Mice. Intern Med. 12:362.
Received: 04-Apr-2022, Manuscript No. IME-22-16667; Editor assigned: 06-Apr-2022, Pre QC No. IME-22-16667; Reviewed: 20-Apr-2022, QC No. IME-22-16667; Revised: 25-Apr-2022, Manuscript No. IME-22-16667; Published: 11-May-2022
Copyright: © 2022 Wang H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.