Andrology-Open Access
Open Access
ISSN: 2167-0250
ISSN: 2167-0250
Mini Review - (2023)Volume 12, Issue 4
Two global tendencies: The decline in male fertility and increase in cancer are very alarming. Here we present the extended Mini-Review of the recently published article and some literature references analyzing the reciprocal link (ouroboros) of both pathologies. We showed that it was rooted in multicellular evolution and became enhanced by current environmental pollution and endocrine disruption. The findings are based on the phylostratigraphic analysis of the networked functional modules of ~1500 gametogenic genes which have evolved from Unicellulars to Homo sapiens. The evolutionary key to the link for male reproduction and cancer was revealed in the multicellulars of the Cambrian explosion (~500 Mya) when innate immunity/inflammation (including the proto-placental genes) and hormonal sex determination emerged, shared and hubbed by stress-response genes FOS/JUN and cytokine IL1β. The Achilles heel of male sex determination is hidden in its archetypal male-female reversal, triggered by stress and endocrine disruption. This reversal likely redirects tissue proliferation towards the mito-meiotic transition creating the pseudo-parthenogenetic Polyploid Giant Cancer Cells (PGCCs). The latter firstly evolved in unicellulars and early metazoans as part of sporogenesis and embryogenesis. Reciprocally, somatic sex reversal causes suppression of spermatogenesis, strengthened in late mammalian evolution by Cancer Testis Associated Genes (CTA/MAGEA group) in collaboration with testosterone-responsive androgen receptors. In addition, the placental syncytin glycoproteins, created by mammalian domestication of endogenous retroviruses and employed in cell fusion and immunity suppression, can cause fusion of cancer and somatic cells for the organ-targeted metastases. Alternatively, both germinative and the cytotrophoblast components can differentiate within a single multinucleated PGCC.
Polyploid Giant Cancer Cells ( PGCCs); Male infertility; Somatic sex determination; Sex reversal; Cancer testis antigens; Androgen receptor; Immunity; Endogenous retroviruses; Cancer resistance; Metastasis
Two very alarming tendencies are threatening humankind: the global decline in male fertility, documented over 80 years of monitoring, and increase in cancer; both are accelerating in the 21st century shown in Figures 1A and 1B [1,2]. Moreover, cancer incidence is currently rising in adults under 50 and prostate cancer in younger men has increased in most countries [3,4]. Furthermore, men are more likely to both develop and die from malignant tumours; azoospermic men have an up to a 3-fold higher risk of any cancer than fertile men [2,5].
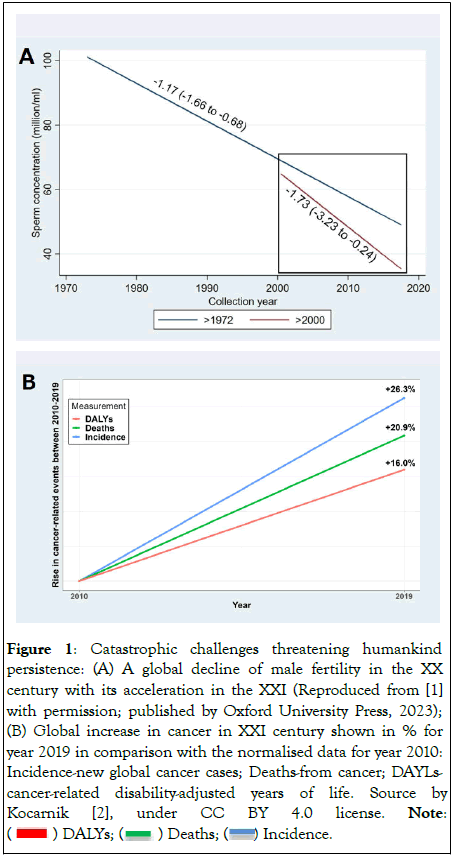
Figure 1: Catastrophic challenges threatening humankind persistence: (A) A global decline of male fertility in the XX
century with its acceleration in the XXI (Reproduced from [1]
with permission; published by Oxford University Press, 2023);
(B) Global increase in cancer in XXI century shown in % for
year 2019 in comparison with the normalised data for year 2010: Incidence-new global cancer cases; Deaths-from cancer; AYLscancer-
related disability-adjusted years of life. Source by
Kocarnik [2], under CC BY 4.0 license. Note: ( ) DALYs; (
) DALYs; ( ) Deaths; (
) Deaths; ( ) Incidence.
) Incidence.
While it can be reasonably speculated that both disasters are caused by similar adverse conditions, like metabolic syndrome, the HIV epidemic, etc., in the recently published article the hypothesis of the reciprocal link of both pathologies rooted in remote evolution and enhanced by current environmental pollution was analysed [6].
One of the prerequisites for this assumption is the long-standing (established in the 19th century) embryological theory of cancer (“Cancer is a Bad Embryo”) in its parthenogenetic variant and the current twist on this theory, highlighting tumour polyploidy and equalising Polyploid Giant Cancer Cells (PGCCs) typical of aggressive tumours with blastulae enabled by mito-meiotic somagerm transition as a parthenogenetic mechanism of carcinogenesis [7-11]. Another rationale stems from the works showing wide expression of germ cell genes in malignant tumours, as well as the association between polyploidization (whole-genome duplication) and enrichment of reproductionrelated functional gene modules [12,13]. The third rationale for seeking the link between male reproduction mechanisms and cancer stems from the oncogenic (poor prognosis-associated) features of Cancer-Testis Antigens (CTA), with the crucial MAGEA group of transcription factors stimulating spermatogenesis in the testis but also ectopically expressed in placenta and cancers [6,14-17].
In our previous work, we took advantage of the phylostratigraphic analysis of the evolutionary origin of ~1500 known Gametogenesis-Related Genes (GG) along the whole 3.9 billion years of Life evolution, from Prokaryota to Homo sapiens [13]. The obtained evolutionary GG profile of human genome gene expression related to all gene origins is presented in Figure 2.
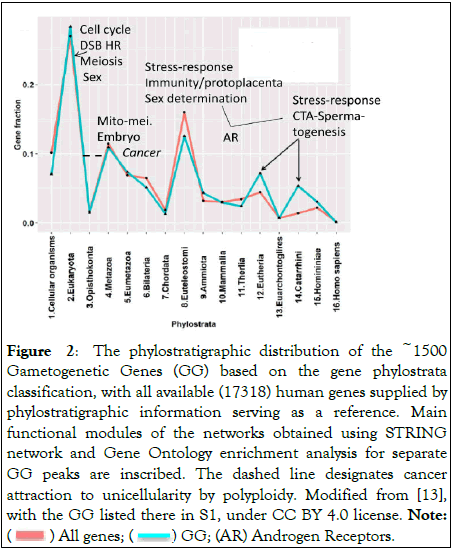
Figure 2: The phylostratigraphic distribution of the ~1500
Gametogenetic Genes (GG) based on the gene phylostrata
classification, with all available (17318) human genes supplied by
phylostratigraphic information serving as a reference. Main
functional modules of the networks obtained using STRING
network and Gene Ontology enrichment analysis for separate
GG peaks are inscribed. The dashed line designates cancer
attraction to unicellularity by polyploidy. Modified from [13],
with the GG listed there in S1, under CC BY 4.0 license. Note:
( ) All genes; (
) All genes; ( ) GG; (AR) Androgen Receptors.
) GG; (AR) Androgen Receptors.
While the peaks of acquisition of new GG genes on the whole corresponded to the evolutionary pace, the group of CTA genes in Eutherians (placental mammals) and Catarrhini (predecessors of hominids) showed two late splashes of disproportional amplification. About 40 families of these genes (transferred from autosomes onto X-chromosome by mobile genetic elements about 170 Mya and amplified there) are mostly responsible for spermatogenesis in male gonads associated with the stress response [18]. The study of GG gene expression in TCGA database cancer transcriptomes revealed 10 tumour cohorts (BLCA, BRCA, GBM, LIHC, LUAD, LUSC, OV, PRAD, SARC, STAD) possessing CTA-MAGE group genes in their top-25 upregulated genes. These are activated in cancers by unappropriate demethylation of promoters [19].
The following questions arise: (1) why was boosting of spermatogenesis by CTAs needed so late in evolution when the reproductive process was already well-established and (2) why and how is male reproduction related to cancer? The reasonable considerations found in literature are as follows: the developed brain of the mammals competed for energy with male reproduction, weakening it and creating a need for its reinforcement and protection from stress (e.g. hunting, famine, social stress). The way how this was done in the late mammalian evolution (by mobile retroviral element transposition of CTA) created X-chromosome fragility at the sites of CTA insertions, inducing predisposition to genome instability and gene diversification, which are still going on at these hot spots [6,18].
In the work by Erenpreisa and colleagues the evolutionary phylostratigraphic peaks of GG genes were further explored in the Homo sapiens transcriptome in more detail, deciphering the functional modules of the networks enclosed in these peaks, using STRING protein-protein interaction networks and Gene Ontology module enrichment analysis [6]. The obtained reproductive modules, presented in the original article by gene networks are shown Figure 2.
It can be seen that the response to DNA damage (DNA Double Strand Breaks DSBs) and its repair by Homologous Recombination (HR), which gave rise to meiosis and sex, appeared already in unicellular Eukaryotes; the separation of soma and germ (mito-meiotic transition) occurred in the early multicellular organisms, giving rise to the embryo. Interestingly, the literature sources show that the first cancer (in the Eumetazoan hydra) and cancer-related protooncogenes emerged simultaneously with these processes, along with cancer attraction to unicellularity by polyploidy indicated by a dashed line shown in Figure 2 [20-25].
But where is the relationship between cancer and male infertility? Two recent articles revealed a key to this puzzle [6,26]. The STRING network analysis of the 8th Phylostratum from the Cambrian animal explosion (Euteleostomi, ~500 Mya) which also produced the peak of GG genes, revealed in the human genome the somatic regulation of reproductive processes shown in Figures 2 and 3. One of the functional modules is represented by an innate immunity/inflammation network of transcription factors and secreted cytokines hubbed by interleukin 1 beta (Il-1β). This subnetwork as it turned out, incorporates ten genes comprising the gene ontology module “Female Pregnancy” (GO:0007565) – employed in stress, cell fusion, invasion, and vascularisation (FOS, JUNB, VEGFA, IL1β, THBD, AREG, AGT, PGF, PTHLH, and STC2).
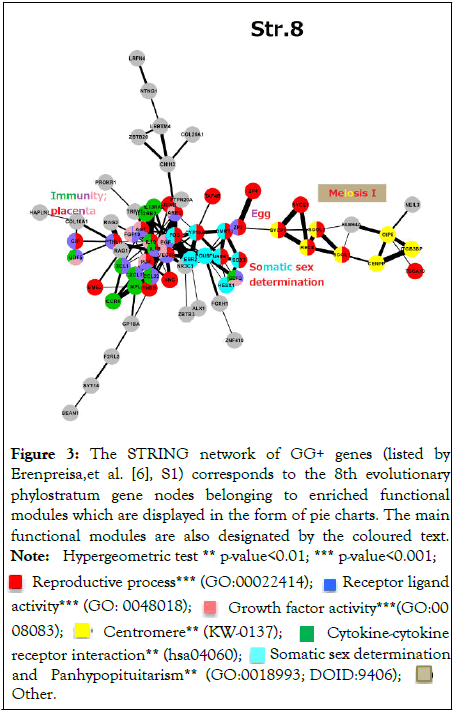
Figure 3: The STRING network of GG+ genes (listed by
Erenpreisa,et al. [6], S1) corresponds to the 8th evolutionary
phylostratum gene nodes belonging to enriched functional
modules which are displayed in the form of pie charts. The main
functional modules are also designated by the coloured text.
Note: Hypergeometric test ** p-value<0.01; *** p-value<0.001;
 Reproductive process*** (GO:00022414);
Reproductive process*** (GO:00022414);  Receptor ligand
activity*** (GO: 0048018);
Receptor ligand
activity*** (GO: 0048018);  Growth factor activity***(GO:0008083);
Growth factor activity***(GO:0008083);  Centromere** (KW-0137);
Centromere** (KW-0137);  Cytokine-cytokine receptor interaction** (hsa04060);
Cytokine-cytokine receptor interaction** (hsa04060);  Somatic sex determination
and Panhypopituitarism**
Somatic sex determination
and Panhypopituitarism**  (GO:0018993; DOID:9406);
Other.
(GO:0018993; DOID:9406);
Other.
Another module is of somatic sex determination governed by pituitary hormones. Notably, both somatic modules involved in reproduction were found united and shared by the stress response cassette AP-1 (a dimer of FOS and JUN transcription factors families) and linked to egg maturation and meiosis (Figure 3). The Phylostratum 8 was also found dramatically rocketting with differentially expressed genes in the experimental model of drug-resistant breast cancer [6,26,27].
And here, the Achilles heel of sex determination critical for our puzzle is hidden shown in Figure 3. In short, somatic sex determination is inherently favourable for female development, while for male development, it needs strict genetic and epigenetic tuning which is dosage-sensitive to stress [6]. Stress shuttles this system towards male-female reversal in-utero based on testosterone-oestrogen conversion and the corresponding regulation of androgen-oestrogen-progesterone nuclear receptors (AR-ER-PR) (well known to andrologists and detailed in the related article) [6].
In a reproductive context, the master stress gene FOS is critical for upregulation of key ovulatory prostaglandins in human granulosa cells, mediated through Progesterone Receptor (PGR) and Epidermal Growth Factor (EGF) signalling, while progesterone activates oocyte maturation, prepares endometrium epithelium and favours embryo implantation [28,29]. This makes FOS part of the ‘Female Pregnancy’ module and of sex reversal. AR receptors of the somatic sex determination system, normally responsive to testosterone produced in the testis by Leydig cells, are found as proteins and/or transcripts both in male and female reproductive organs, including the endometrium, in many somatic tissues (breast, prostate, kidney, urinary bladder, brain) and also in somatic cancers, possibly, being involved in mito-meiotic transition initiating or accompanying oncogenesis [30].
In Phylostrata 10-16, the spermatogenic CTA/MAGEA genes which amplified on the X-chromosome in late mammalian genomes are also stress-responsive and dose-sensitive [18]; moreover, some (MAGEA1 and MAGEA11) are linked to and collaborating with the somatic reproduction regulators of the 8th phylostratum through the AR receptor shown in Figure 2.
Modern world is characterised by global, local, and individual environmental pollution with different chemicals that act as endocrine disruptors. Many of them (like phthalates, parabens, bisphenol A, pesticides, cosmetic hormonal components, and others) are either disrupting the development of the male reproductive system in-utero (through maternal exposure), and/or are exhibiting a negative effect on spermatogenesis postnatally and in adulthood, due to their estrogenic or antiandrogenic effect [31]. In effect, the level of testosterone in adolescent and young adult men as monitored in the USA between 1999 to 2016 was evidenced as decreasing by 1% per year [32]. Environmental pollution in combination with a shift to a more unhealthy life-style (lack of physical exercise, junkfood, etc.) is usually considered to be the essential causing factor for the decline in male fertility [33].
The reproductive modules of the whole 3.9 billion year-long human evolution, from unicellular Eukaryotes till Homo sapiens, are networked and interdependent. The alarming tendency of decline in male fertility and increase in cancer, which accelerated in the 21st century, was already evolutionary rooted in Euteleostomi by somatic sex determination (~500 Mya) and further in placental mammals, due to the energy competition of male fertility with brain, which was taking place ~170 Mya. The Achilles heel of male sex determination leading to activation of female reproductive pathways in response to stress and endocrine disruption, may favour soma-germ transition in male tissues, resulting in cancer through the parthenogenetic development of PGCCs. The ouroboros of mankind is stress-forced to fuel evolutionary “Female Reproduction-Linked” cancer by sacrificing male fertility (Figure 4).
Figure 4: The symbolic Ouroboros of humankind forced by stress and endocrine disruption to fuel metastatic cancer by sacrificing male fertility.
Therefore, a very recent work from the laboratory of Jinsong Liu using the contraceptive drug mifepristone (progesterone inhibitor) suppressing in combination the acquired resistance of PGCCs to conventional treatment seems a logical and perspective approach to anticancer treatment [34]. For Proof of the Ouroboros Principle, it is also interesting to highlight the recent data that revealed that castration-resistant prostate cancer can be killed by a testosterone shock [35], and also the work on suppression of Il-1β for overcoming resistance of PGCCs [36].
The current decline of male fertility driving increase in cancer rooted in mammalian evolution and accelerated by environmental pollution, can be described by the Greek mythological Dragon Ouroboros eating its own tail. This fatal switch running metastatic cancer may be possibly defeated by drugs preventing oocyte maturation and embryo implantation.
Zina Luft is acknowledged for the idea of Mythologic Ouroboros.
No institutional funding.
The authors declare no conflict of interests.
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Erenpreisa J, Vainshelbaum NM, Salmina K, Zayakin P, Erenpreiss J (2023) The Ouroboros of Human Evolution: Global Decline of Male Fertility and Increase in Cancer. Andrology. 12:293.
Received: 21-Aug-2023, Manuscript No. ANO-23-26181; Editor assigned: 24-Aug-2023, Pre QC No. ANO-23-26181 (PQ); Reviewed: 07-Sep-2023, QC No. ANO-23-26181; Revised: 14-Sep-2023, Manuscript No. ANO-23-26181 (R); Published: 21-Sep-2023 , DOI: 10.35248/2167-0250.23.12.293
Copyright: © 2023 Erenpreisa J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.