
Biochemistry & Pharmacology: Open Access
Open Access
ISSN: 2167-0501

ISSN: 2167-0501
Research Article - (2014) Volume 3, Issue 6
The purpose of this investigation was to study the effects of gold (Au) and silver (Ag) nanoparticles on a commonly used enzymatic reaction between horseradish peroxidase (HRP) and the substrate, 3,3',5,5'-tetramethylbenzidine (TMB). Two different methodologies were used in this study. The first was the addition of small quantities of nanoparticles, directly onto a solution of streptavidin-peroxidase (streptavidin bound to HRP), prior to its reaction with TMB. The second was the addition of nanoparticles to immobilized streptavidin-peroxidase, covalently bounded to wells of a microtitre plate. In both cases, reactions with TMB were measured by visible absorbance spectroscopy, using a microtitre plate reader. The results indicate that both gold and silver nanoparticles have a significant effect; with gold suppressing the reaction and silver enhancing it.
<Keywords: Nanoparticles, Horseradish peroxidase, Tetramethylbenzidine
The reaction between the enzyme, horseradish peroxidase (HRP), and the substrate, 3,3',5,5'-tetramethylbenzidine (TMB), is well established and frequently used in Enzyme-Linked Immunosorbent Assays (ELISAs) for detecting and measuring analytes, such as proteins and antibodies. HRP has a metal ion cofactor, known as haem, which is a prosthetic group consisting of an iron ion. When haem, in the peroxidase, is combined with TMB, it produces a one-electron oxidation product, which is a free radical cation that is responsible for the change in colour from an almost colourless solution to dark blue as explained [1]. The degree of this colour change is directly reflective of the amount and activity of the enzyme; hence its use in ELISAs as the signal generating stage. When an acid is applied to the enzymesubstrate complex, a further reaction takes place, producing a twoelectron diimine product, which changes the colour from dark blue to yellow.
The purpose of the following experiments was to investigate the effects of gold and silver nanoparticles on the HRP activity, using TMB as the substrate. Visible absorption spectroscopy was used to obtain readings of the amount of light transmitted through the wells of a microtitre plate. These readings, known as transmittance data, Io, were then converted into relative absorbance, Ar, using Equation 1:
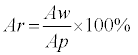 (1)
(1)
Where,
Aw = Absorbance of the contents of a microtitre well
Ap = Absorbance of the contents of a microtitre well with a positive control reference
The absorbance, Aw or Ap, was calculated using the generic equation for absorbance, A, shown in Equation 2:
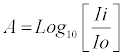 (2)
(2)
Where,
Ii = Intensity of light entering a microtitre well
Io = Intensity of light leaving a microtiter well (transmittance)
Materials
A Hyaluronidase Assay Kit, from Razie Biotech Ltd., was purchased from AMS Biotech.com.
The following materials were purchased from Sigma-Aldrich UK: 3,3’,5,5’-tetramethylbenzidine; streptavidin peroxidase (streptavidin bound to HRP; 1 mg of lypholised powder was dissolved in 1 ml of phosphate buffered saline (PBS) solution, a 100 times (v/v) in PBS was used in the procedure); gold colloid solution, 20 nm; gold colloid solution, 10 nm; gold colloid solution, 5 nm; silver nanoparticles dispersed in water, citrate stabilised, 157 nm.
Apparatus
1. Two, 96-well microtitre plates; one of them coated with biotin
2. Shaker/incubator; set at 390 r.p.m. and 37 ºC
3. Microtitre plate-reader
Experimental procedure
The experimental procedure was carried out using two methodologies, a direct method and a method using a biotin bounded plate. Both methods required streptavidin, dilute 100 times (v/v) in PBS, which was the first task to be performed.
The direct method used six wells of a non-coated, 96-well microtitre plate. Transmittance readings, through the empty wells, were recorded using the plate-reader. A 2 μl drop of streptavidin peroxidase:PBS solution was placed in the centre of one of the wells, which was used as a positive control reference well, i.e. without the addition of any nanoparticles. A 5 μl drop of 20 nm gold nanoparticles was placed in the centre of the second well, which was used as a negative control reference well, i.e. without the addition of any streptavidin:PBS solution. A 2 μl drop of streptavidin peroxidase:PBS solution was placed in the centre of each of the four remaining wells and 5 μl of nanoparticles were added to each of these drops, using the following materials: 20 nm Au nanoparticles were added to the first drop, 10 nm Au nanoparticles were added to the second drop, 5 nm Au nanoparticles were added to the third drop and 157 nm Ag nanoparticles were added to the forth. The plate was placed in the shaker/incubator for a period of 10 minutes. It was then removed from the shaker/incubator and each of the six wells were filled with 50 μl of TMB, prior to the plate being returned to the shaker/incubator. Another set of transmittance readings were recorded after 40 minutes incubation.
The bounded method, used four biotin coated wells of a 96- well, microtitre plate. Each well was filled with 50 μl of streptavidin peroxidase:PBS solution and placed in the shaker/incubator for a period of 30 minutes. The wells were emptied, washed in distilled water and dried, prior to the addition of nanoparticles. One well was left void of nanoparticles and used as a positive control reference well. The remaining three wells were filled with 50 μl of nanoparticles. The first with 5 nm Au nanoparticles, the second with 20 nm Au nanoparticles and the third with 157 nm Ag nanoparticles. The microtitre plate was returned to the shaker/incubator for a period of 10 minutes; then, emptied, washed with distilled water and dried again. Transmittance readings, of the empty wells, were recorded using the plate reader, prior to filling each well with 50 μl of TMB. The plate was returned to the shaker/incubator for a further 30 minutes, and then another set of transmittance readings were recorded.
Both sets of transmittance readings, obtained from each of the above procedures, were used to calculate the absorbance of the contents of each well, relative to the absorbance of the positive control reference wells, using Equations 1 and 2.
The procedures were repeated a number of times to obtain mean values.
For the direct method, no colour change was detected in the negative control well, where 20 nm gold nanoparticles were added to TMB on its own.
The results, expressed for each well, are the mean values of six wells, in three sets of experiments
When the wells were observed by eye, both experiments revealed that only the positive control reference wells and those containing silver nanoparticles appeared to be dark blue in colour; the remaining wells appeared to be either transparent or a very pale blue.
The results, obtained from the two different methodologies, indicate that gold and silver nanoparticles have a significant effect on the enzymatic reaction between HRP and TMB.
The positive control reference wells contained the enzymesubstrate complexes only, without any nanoparticles. These were expecte to produce normal reactions and the relative absorbance of these complexes was used as reference points of 100%.
In the direct method, a 5 μl drop of 20 nm gold nanoparticles was placed in the centre of the negative control reference well, without the addition of any streptavidin:PBS solution. This was expected to produce no reaction and the relative absorbance of the nanoparticle/TMB solution was used as a baseline reference (i.e. the minimum amount of relative absorbance). The results clearly show that the nanoparticles did not react with the TMB and played no part on the conversion of TMB into colour products. The addition of streptavidin:PBS solution, on the other hand, together with the same size gold nanoparticles, provides evidence that colour products were produced by the reaction between streptavidin:PBS and TMB alone, while the gold nanoparticles inhibited this reaction by almost 40% relative absorbance (Figure 1).
The direct method also provides evidence that biotin, which was coated on the microtitre plate for the bounded method, had no part to play in the reactions. The role of biotin was only to bind HRP to the walls of the microtitre wells.
In both experiments, the relative absorbance of the enzymesubstrate complex, containing silver nanoparticles, exceeded that of the complex contained in the positive control reference well. Thus, indicating that the silver nanoparticles enhanced the reaction between HRP and TMB [2]. Who demonstrated that silver nanoparticles not only enhances the activity of HRP, but also alters its structure. However, their results also indicate that this only occurred within a specific concentration range of silver nanoparticles (Figure 2).
On the other hand, the relative absorbance of the enzymesubstrate complex, containing gold nanoparticles, was less than that of the complex contained in the positive control reference well. Thus, indicating that the gold nanoparticles inhibited the reaction between HRP and TMB. Furthermore, the results also show that these reactions appear to be influenced by the relative size of the nanoparticles, with 20 nm gold nanoparticles having the least effect and the smaller nanoparticles exhibiting greater inhibition.
Considering these results, it is not unreasonable to suggest that other enzymes may also be affected by nanoparticles, and that different types of nanoparticles, of different shapes and sizes, may affect enzymatic reactions, in different ways [3], has shown that the properties of nanoparticles, such as size, shape, surface chemistry and charge, can alter the structure and function of enzymes [4], has also demonstrated that nanoparticles are able to induce protein modifications.
A great deal of research work is being carried out on the effects of nanoparticles on biological systems. It comes as no surprise that these systems are influenced by nanoparticles, since the common ground between nanoparticles and biology is the nanoscale [5], has demonstrated that gold and silver nanoparticles could enhance the radiation sensitivity of hepatocellular carcinoma cells (HCC), a common type of liver cancer. The mechanism for this is thought to be due to programmed cell death (apoptosis) or elevated DNA damage.
Veeraapandian demonstrated that protein capped gold nanoparticles have been shown to exhibit good antioxidant activity, which reduces cell damage or death, while protein capped silver nanoparticles are able to inhibit the growth of several Gram-positive and Gram-negative microorganisms [6].
Enzymatic reactions are influenced by nanoparticles. The mechanism of these nanoparticle-enzyme interactions are currently under investigation. This is an important area of research, since the ability to regulate enzymatic activity could potentially lead to new processes for enzyme control and modification, with possible use in new drug developments and industrial applications. It also signifies probable toxicity of nanoparticles as they become more widely present in everyday life via off-the-counter available cosmetics and household goods.