Journal of Chemical Engineering & Process Technology
Open Access
ISSN: 2157-7048
ISSN: 2157-7048
Research Article - (2019)Volume 10, Issue 1
This paper addresses the stability of commercial cation exchangers when in contact with nitric acid that are under consideration for UCLan’s alternative reprocessing process. The paper describes the acid tolerance of four commercial ion exchange resins when exposed to 7M nitric acid for one year. The acid degradation was measured by FT-IR and TGA before and post acid exposure. Zirconium uptake was measured pre and post acid exposure as a means of determining degradation of functional sites. The resins undergo a degree of acid degradation depending on their chemical composition i.e., polymeric backbone, in some instances loss of functionality but also the introduction of new functional sites. The likely radiation dose that ion exchange resins would receive from spent nuclear fuel dissolver liquor and from adsorbed radioactive cesium 137 were estimated. It was inferred that the radiation dose the exchangers would receive from adsorbed cesium isotopes would be comparatively low when compared with the radiation background of the spent fuel dissolver liquor.
Commercial ion exchange resins; Degradation; Acid/ radiation stability; Cation uptake; Zirconium
UCLan has previously published a new approach to spent fuel reprocessing [1], which is based on the sequential chromatographic selective separation of fission products and minor actinides from uranium and plutonium. The UCLan concept would initially remove the cesium and strontium isotopes (β/γ emitters) significantly reducing the heat load on downstream stages and the arising of highly active waste. The cost of reprocessing and waste disposal could also be reduced significantly.
Cationic resins have an arsenal of versatile properties, which contributes to their wide use in various industrial processes such as in catalysis, chemical and nuclear industry separations [2]. Under normal applications, these resins, in particular the sulphonic acid, exhibit high exchange capacity, but are limited with their thermal instability [3]. In our case the stability in nitric acid solutions containing fission products that are undergoing decay and emit γ radiation will be crucial for both process and storage considerations.
The use of ion exchange resins for separating fission products and minor actinides from uranium and plutonium present in spent nuclear fuel dissolver liquor has not been previously attempted. This is in part due to the industry’s preference for the PUREX process, which has remained unchallenged for more than sixty years. The PUREX process has not only demonstrated its efficiency in achieving the required decontamination factors needed to produce recyclable uranium and plutonium but also the relative stability of the main components (tri butyl phosphate and diluent) to nitric acid and radiation. It is crucial therefore that candidate replacement processes will have to match, if not exceed these attributes. Our previous paper explored the affinity of commercial ion exchange resins for fission product separation [4]. This paper explores the compatibility of four of these candidate resins in 7M nitric acid, their thermal stability and predicts the radiation that they would have to endure on exposure to spent fuel dissolver liquor and on adsorbing fission products from this solution and compares these predictions with previous studies [5].
Materials
The four resins, selected for this work were C100H (sulphonic acid, polystyrene 8% cross-linked with divinyl benzene), C100 × 10MBH (sulphonic acid, polystyrene 10% cross-linked with divinyl benzene), S910 (amidoxime, polyacrylic cross-linked with divinyl benzene) and D5530 (inorganic material of unknown composition and structure). These four materials were chosen based on their affinities and selectivities for fission products and cerium as surrogate for U/Pu, which have been reported in the previous paper [4]. All four resins were provided by PUROLITE Ltd and used without any prior treatment.
Acid stability studies
The four exchangers were exposed for about one year to 7M nitric acid, this concentration is substantially higher than that normally associated with the PUREX process (2-3M), but was used to accelerate the degradation thus providing useful information. The stability was assessed by comparing the Zr ion uptake of the as received exchanger with acid exposed. This ion uptake comparison was complemented by infra-red analysis (FT-IR) of dried, crushed unexposed and exposed exchangers.
Ten grams of the four target exchangers (C100H, C100 × 10MBH S910 and D5530) were placed in a 250 mL Duran bottle with 250 mL of 7 M HNO3 for a year. They were rotationally shaken for an hour three times a week at 200 rpm and inverted weekly.
A 0.5 g sample of as received and nitric acid treated resin was equilibrated with 100 ml of Zr solution (Zr 500 ppm) for 24 hours. The Zr concentration of the solution was determined before and after equilibration using ICP-MS as described in the previous paper [4].
Thermal degradation
The thermal degradation of three of the exchangers (~100 mg quantities) was studied using Mettler Toledo TGA 1 with a heating rate of 10°C min-1 in air (50 mL min-1). The percentage weight loss with temperature (°C) was recorded.
Radiation exposure
In situ radiation experiments although desirable was not possible at UCLan, exposure to external sources such as a Co-60 was considered but as previous work [5] had not produced conclusive evidence this was not further explored; however the potential for irradiation damage to the exchangers by fission product decay was assessed.
Ionising radiation doses in the range of 105 to 106 Gy significantly alter the properties of most organic ion exchangers. High-absorbed doses affect exchange capacity, selectivity and exchange kinetics; other physical and chemical properties are also affected. In general, anion exchangers suffer more radiation damage than do cation exchangers [6]. Radiolysis effects on ion exchangers depend on the nature of the resin itself, its chemical composition, ionic form, moisture content, swelling characteristics and the extent of cross-linkage in the macromolecular structure [7] cf thermal and chemical degradation. The type of radiation and the resin environment also affects radiolytic changes during irradiation [8].
The potential for irradiation damage to the exchangers by adsorbed fission products (Cs) was calculated using the following eqn. [9]:
where:
Dose is in Gy
M is the activity in Bq.
ϵ is the absorption cross section for ion exchange resin (πr2, metres).
E is the gamma energy in MeV.
t is the exposure time in seconds.
r is the distance from source in m.
is density of the exchanger kg/m3.
The calculated values were cross-checked using the Nucleonica Mass Activity Converter [10].
Acid stability
Zr in 2M nitric acid was selected as the ion uptake barometer for checking the degree of acid degradation as the ion exchange materials had the greatest affinity for this ion compared to Cs and/or Sr [4]. The Zr ion uptake values and moisture content of as received and acid treated materials are reported in Table 1.
| Resin | Repeat | Dry mass (%) | Wet resin Zr capacity (mg/g) | Average | |
|---|---|---|---|---|---|
| wet resin Zr capacity (mg/g) | Dry Zr capacity (mg/g) | ||||
| C100H has received | 1 | 63.2 | 15.7 | 16 | 25.3 |
| 2 | 16.3 | ||||
| C100H acid exposed | 1 | 42 | 11.9 | 11.85 | 28.2 |
| 2 | 11.8 | ||||
| C100 × 10MBH | 1 | 68.9 | 14.5 | 14.35 | 20.8 |
| as received | 2 | 14.2 | |||
| C100 × 10MBH | 1 | 42.8 | 12.2 | 12 | 28.1 |
| Acid exposed | 2 | 11.9 | |||
| S910 has received | 1 | 57.4 | 3 | 3 | 5.24 |
| 2 | 3.1 | ||||
| S910 acid exposed | 1 | 27 | 12.3 | 12.5 | 46.3 |
| 2 | 12.7 | ||||
| D5530 has received | 1 | 62.5 | 4.4 | 4.35 | 7 |
| 2 | 4.3 | ||||
| D5330 has received | 1 | 60.6 | 2.1 | 1.85 | 3.1 |
| 2 | 1.6 | ||||
Table 1: Effect of extended exposure to 7M HNO3 on four ion exchangers’ Zr capacities.
Nitric acid stability of ion exchange resins has been studied on numerous, previous occasions, but many of these studies involved anion exchangers [11]. Fewer studies have concentrated on cation exchange resins, but some have involved sulphonic exchange materials [12]. The oxidative decomposition of sulphonic acid resins whether due to nitric acid, thermal or radiation tends to follow a systematic and common path namely;
• The sission of sulphonic acid groups leading to the formation of sulphuric acid, followed by
• Oxidation of the polymeric backbone [13]
The latter reaction results in the decrease in the cross linking and ion exchange capacity but increases the moisture content and degree of swelling [13]. The studies also demonstrated that the hydrogen form of sulphonic acid resins was in general less resistant to oxidative attack but anionic exchangers were unquestionably more readily oxidised [14].
The results gathered in this study are consistent with previous work in that:
• Moisture content of acid exposed organic ion exchange resins is higher than the untreated material; for the sulphonic acid resins (C100H and C100 × 10MBH) the moisture content value increased by about 50% (~40% to ~60%). This is consistent with data produced by Purolite, but it used a solution of hydrogen peroxide (1%) and iron at 40°C but for only a few days exposure [15]. As the Zr capacity for the C100H resin has not been significantly affected (~11%) by 7M nitric acid exposure when comparing dry mass values (Table 1), then the loss in weight could arise from the release of polymer leachables coupled with the oxidation of the polymer backbone producing more polymer leachables. The Zr capacity for acid exposed C100 × 10MBH resin however increased, based on dry mass weight, by about 35% but this increase can be directly attributed to the difference in moisture values.
• The degree of cross linking (cf C100H with C100 × 10MBH) slightly influenced moisture levels of treated resins when compared with as received material.
• The moisture content of D5530 material was relatively unaffected on exposure to acid but its capacity for Zr ions was reduced by about 60% (comparing dry weight values) after exposure. As this material was supplied without technical details it is not possible to provide a satisfactory explanation other than some of the functionality was lost on exposure to 7M nitric acid solution.
• Both moisture values and Zr capacity of S910 amidoxime resin were significantly affected by nitric acid exposure (Table 1). The moisture levels of the acrylic polymeric material changed from ~33% to ~63% after exposure but with the Zr capacity increasing 9 times (based on dry weight). Only ~20% of this increase can be attributed to the change in moisture content, which leaves ~80% attributed to changes to the functional group and/or polymeric backbone. The instability of the amidoxime resin is consistent with previous published data; Vernon demonstrated that a resin prepared in his laboratory lost 50% of its capacity having been exposed to 0.1 to 1.0M acid for 7 days [16]. Argonne National Laboratory has reported that 50% destruction of aceto-hydroxamic acid (AHA) in 1M acid after 338 minutes substantiates our observation [17]. Other studies have reported the hydrolysis of amidoxime functional group in the presence of strong acid leads to a polymer containing simultaneously hydroxamic acid, amide and carboxylic acid groups [18]. This mechanism would lead to an increased capacity but may not be sufficient to explain the full extent of improved capacity and therefore the oxidation of the polymeric backbone to produce more functional sites, such as – COOH could have occurred which would also account for the mass loss.
FTIR spectra
The IR spectra of the sulphonic acid resins in the 3700-500 cm-1 region are shown in Figures 1 and 2. FTIR spectral analysis shows a series of peaks in the 1000-1100 cm-1 region which are due to SO3 symmetrical stretching; O-H stretching in the 2900-2400 cm-1; S-O stretching at ~670 cm-1; C=C aromatic nucleus skeletal vibration band at 1550-1670 cm-1 and OH hydrogen bond broad stretching band at 3200-3500 cm-1. The spectra for the two C100H samples show extremely strong resemblance (Figure 1), but the dry mass results in Table 1 indicate that after nitric acid exposure 50% of the dry mass has been lost without loss of capacity. As suggested, earlier this weight difference could be attributed to release of polymer leachables.
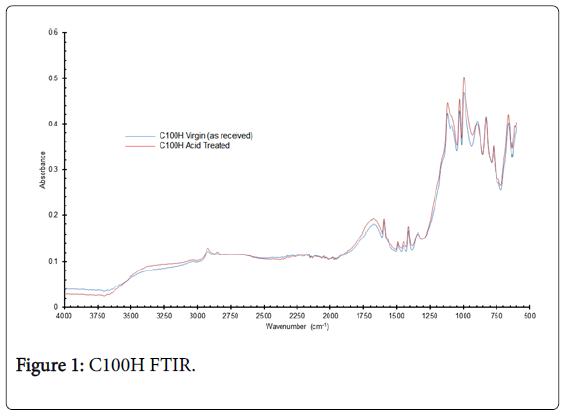
Figure 1: C100H FTIR.
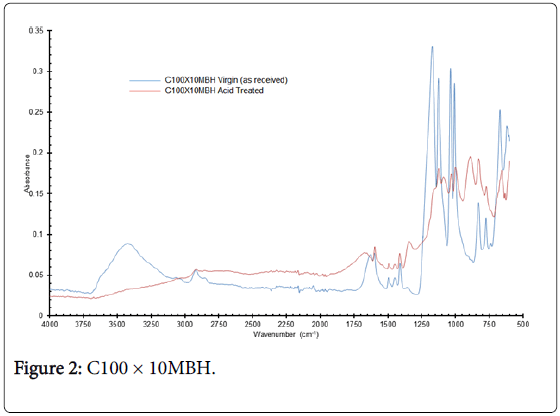
Figure 2: C100 × 10MBH.
The C100 × 10MBH spectra (Figure 2) show some distinct differences, for example loss of peaks at 3400 cm-1 and 1590 cm-1 with a shift in peaks from 833 to 881 cm-1 and 770 to 822 cm-1 for the as received compared with acid exposed resin. These differences can be attributed to changes to either functional group or possibly with the organic structure leading to differences in vibration energies. The spectra for D5530 materials are comparable, but unfortunately, it is not possible to assign with confidence analysis to the various peaks. Hydrogen bonding stretching could be associated with the broad peak at 3250 cm-1; the intense peak at 1045 cm-1 may be due to a variety of groups ranging from S, P and N to SiO2.
The resin that had undergone the most radical change of its structure and composition is S910 amidoxime resin. The characteristic amidoxime peaks and assignments are reported in Table 2.
| Wave Number (cm-1) | Mode | Acid exposed S910 |
|---|---|---|
| 2917 | CH3 stretching | Lost/diminished |
| 2849 | CH2 stretching | |
| 1647 | C=N stretching | Lost/diminished |
| 1552 | N-H bending | Lost/diminished |
| 1472 | CH3 CH2 bending | Lost/diminished |
| 1392 | C-N stretching | Lost/diminished |
| 934 | N-O stretching | Lost/diminished |
Table 2: Wave numbers and vibrational modes for amidoxime functional group.
After acid exposure of S910, the characteristic amidoxime peaks either were diminished and/or completely removed (Table 2), whereas new peaks appeared at 2350, 1680, 1150 and 770 cm-1. These new peaks could be ascribed to the formation of acrylic acid groups post acid exposure, which could also account for increased Zr capacity of S910 (Figures 3 and 4).
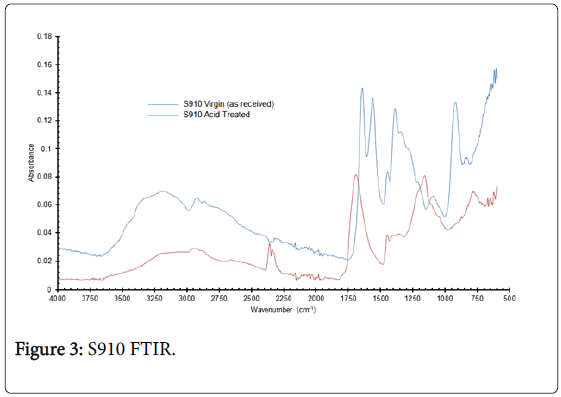
Figure 3: S910 FTIR.
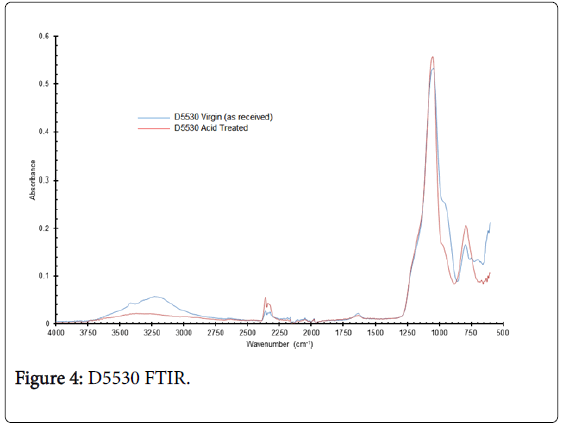
Figure 4: D5530 FTIR.
TGA analysis
The thermo-gravimetric curves of three of the has received resins are displayed in Figure 5; not unexpected significant weight loss (about 30 to 60%) occurs under 200°C which can be attributed to the moisture content. Above this temperature the resins behave differently with D5530 showing very little further weight loss which can be attributed to its inorganic nature. S910 shows the greatest weight loss of nearly 95% at 600°C. The first weight loss (~55%) up to ~160°C is due to the removal of absorbed water molecules; the second stage from 160°C to ~420°C a further 25% is lost due to the further release of water as a result from carboxylic acid groups. From this temperature to ~530°C the polymeric structure begins to decompose, with a total weight loss of ~95% at 600°C.
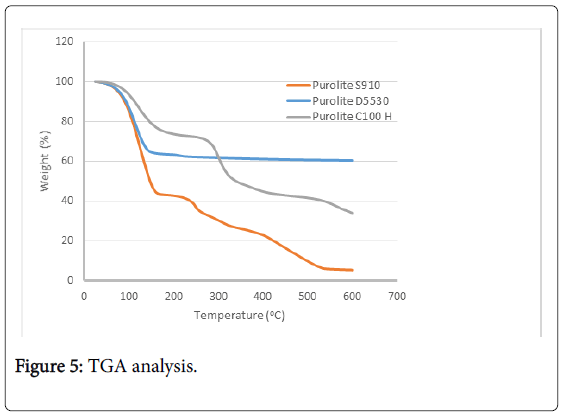
Figure 5: TGA analysis.
The C100H profile is typical of a sulphonic acid resin; initial loss due to moisture followed by the degradation of sulphonic acid groups (loss of oxides of S) ultimately with the degradation of the styrene backbone.
It is extremely unlikely that the resins would experience a temperature greater than 200°C, during processing of spent fuel dissolver liquor more than likely nearer to 60°C and even during storage not above 120°C.
Radiolysis
Several papers in the literature have shown that radiation damage of ion exchange resins could depend on the absorbed dose, the dose rate and the irradiation atmosphere, and that the combination of these factors leads to different results according to the nature of the resin [19]. Moreover, several studies reported that gamma irradiation at doses exceeding 0.1M Gy mainly altered the properties of ion exchange resins, principally anion resins (cf acid oxidation) [20]. Gamma radiation initially leads to direct radiolysis resulting in the scission of functional groups and the formation of radical products or to an indirect radiolytic effect through interactions, which could occur, between the resin and its degradation products such as sulphuric acid for sulphonic acid exchangers. Observations include the release of interstitial water, weight loss, decreased exchange capacities, generation of radiolytic gases, and degradation of the resin matrix [21].
The irradiation of cation resins generally involved the cleavage of – SO3H functional groups, the formation of free radicals and acid products.
The theoretical dose was calculated to demonstrate that low Cs loading capacities from 2 to 3M nitric acid solutions by the sulphonic acid resins under investigation should not lead to a dose greater than 0.1M Gy.
Cs was selected as target radionuclide as its isotopes have by far the greatest γ activity and highest γ energy of the fission products under evaluation in this study.
Using the equation
and the following assumptions:
M=2.405 Bq for 5 μg of Cs absorbed on to 1 g of resin
ϵ=0.4418. 10-6 metres
E=1.6 MeV
t=2.88. 104 seconds
r=10-3 metres
=750 kg m-3.
These values indicate the resin would receive over an 8-hour period 83.06 μGy. This value compares with 39.35 μGy predicted by the Nucleonica mass activity converter. This variation in values can be ascribed to difference in values such as density, absorption cross section as the mass activity converter is set up to predict dose/dose rate to tissue [10].
To achieve a 0.1 MGy dose from Cs isotopes the resin would have to absorbed 5.9 kg/g (~5. 1016 Bq), obviously an impractical value. A more realistic maximum value would be 250 to 300 mg/g, this concentration would produce only 5 Gy dose to the resin. The resin, however, would be subjected to a significant greater dose if the irradiation from the spent fuel dissolver liquor was considered. Assume the radioactivity of the liquor is () 3.7. 1018 Bq [22] with 2.18 MeV (gamma energy for Pr-144) and all other values are the same as above, then the resin could receive a dose of 1.74. 106 Gy. At this dose the resin would be expected to undergo cleavage of functional groups i.e., sulphonic acid and subsequent disruption of the polymeric backbone. This resin’s degradation could be exacerbated by a hostile chemical environment such as 2M nitric acid as the acid will undergo γ radiation producing products that could be more aggressive to the polymeric structure. This will require evaluation when better candidate materials have been found.
Implications for a chromatographic separation of spent nuclear fuel
These stability studies have provided some reassurance that a chromatographic separation of fission products from spent fuel dissolver liquor is feasible, but the ion exchange materials investigated may not be appropriate for the removal of Cs and/or Sr. Of the commercial resins evaluated the sulphonic acid resins have shown a superior stability in nitric acid and radiation (based on predictions) and the data reported in this paper are consistent with previously published information. The prediction of potential radiation damage to the resins is based on the adsorption of radionuclides (Cs) on to the resin, which in this case is extremely low (5 μg/g) and should be emphasised that this is only one component of radiation damage. Fission products in the spent fuel dissolver liquor would contribute to this damage and in fact may be a much greater contributor.
Although the stability results reported here are encouraging it should not be overlooked that the capacity of the sulphonic acid resins for Cs and Sr isotopes are extremely low and in fact have poor selectivity when compared with for example Zr as reported in the previous publication [4].
These ion exchangers for fission product separations, other than Cs and Sr, could be of potential use in later stages of the UCLan chromatographic process, when the bulk of the γ activity has been removed.
Although the stability results reported here are encouraging it should not be overlooked that the capacity of the sulphonic acid resins for Cs and Sr isotopes are extremely low and in fact have poor selectivity when compared with for example Zr as reported in the previous publication.
These ion exchangers for fission product separations, other than Cs and Sr, could be of potential use in later stages of the UCLan chromatographic process, when the bulk of the γ activity has been removed.
The authors would like to thank PUROLITE Ltd (Unit D, Llantrisant Business Park, Llantrisant, Rhondda Cynon Taff, Wales, United Kingdom CF72 8LF) for the provision of ion exchange materials and technical support and to the NNL (National Nuclear Laboratory, Central Laboratory, Sellafield, Seascale, Cumbria CA20 1PG) for technical contributions. JDE’s Ind CASE award was funded by both the NNL and EPSRC (EP1501312/1), the authors are grateful for this support.
Citation: Bond G, Eccles H, Emmott JD (2019) The Acid and Radiation Stability of Some Commercial Ion Exchangers. J Chem Eng Process Technol. 10: 394. doi: 10.35248/2157-7048.19.10.394
Received: 26-Nov-2018 Accepted: 24-Apr-2019 Published: 26-Apr-2019 , DOI: 10.35248/2157-7048.19.10.394
Copyright: © 2019 Bond G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.