
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Review Article - (2022)Volume 12, Issue 4
Batten Diseases (BDs) are a group of lysosomal storage disorders characterized by seizure, visual loss, and cognitive and motor deterioration, and represent the most common forms of neurodegeneration in children. By phenotypic screening, we recently discovered a novel accumulation of Globotriaosylceramide (Gb3) in cellular and murine models of two subtypes of BD, namely CLN3 and CLN7 diseases, which together account for almost 60% of all BD patients. We used fluorescent-conjugated bacterial toxins to label Gb3 and developed a cell-based High Content Imaging (HCI) screening assay that resulted in the repurposing of the FDA-approved hit tamoxifen. Interestingly, tamoxifen showed in vitro and in vivo the activity to ameliorate BD phenotypes through a mechanism that, at least in part, involves the activation of the Transcription Factor EB (TFEB), a master gene of lysosomal function and autophagy. Together, these results might represent the first small molecule therapy to treat BDs and, if supported by accurate preclinical studies and clinical trials to determine dose and safety, tamoxifen might offer an unprecedented opportunity for BD-affected children.
Batten Disease (BD); Globotriaosylceramide; Tamoxifen; Neuronal Ceroid Lipofuscinoses (NCL)
The Neuronal Ceroid Lipofuscinoses (NCLs), commonly known as Batten disease (BD), are a group of recessively inherited fatal diseases of the nervous system which typically arise in childhood. Neurodegenerative disorders in children are rare, with BD being the most frequent; the incidence rates of the entire group of 13 genetically distinct NCLs varies between 1:14,000 and 1:67,000 [1-3]. NCLs are rare conditions with no cure, as treatment options that delay or stop disease progression are still missing, and the disease management remains primarily palliative and targeted at controlling the symptoms rather than curing the disease.
CLN3 disease (MIM # 204200) represents the most common form of NCL worldwide, whereas CLN7 disease (MIM # 610951) is one of the most prevalent BD in southern and Mediterranean Europe. CLN3 and CLN7 are both severe diseases characterized by neurologic involvement, with rapid and devastating progression and early mortality. Especially in CLN7, progression is rapid with mental and motor regression, loss of expressive language and seizures developing in the majority of patients early in the disease course. First seizures and further decline of motor and language function appear by age 4½ years (range of 3 to 7 years), in about one year patients are wheelchair-bound and suffer from complete loss of expressive language. Soon after blindness occurs together with loss of swallowing function (tubefed) and patients are completely dependent on families (range of 9 to 11 years) [4]. The disease leads to a premature death at a mean age 11.5 years (range 6.5 to 18 years). The prognosis is poor with most children becoming severely disabled by midchildhood and not surviving to reach adulthood. This has devastating consequences both on patients and caregivers, in terms of quality of life, physical and emotional costs, and on society and healthcare systems, in terms of financial burden. Unfortunately, the onset of this devastating disease cannot be prevented as it is not included in any newborn screening program for metabolic diseases.
Current management strategies focus on symptom relief and palliative care, which can reduce some symptoms but cannot eliminate the progressively worsening effects caused by neurodegeneration. Moreover, due to disease rarity, many clinicians lack experience treating individuals with this disease. Among BDs, only CLN2 relies on an Enzyme Replacement Therapy (ERT) approved by FDA and EMA, based on the intraventricular administration of the lacking enzyme cerliponase alfa [5]. However, except CLN2, all NCLs are currently incurable diseases, with patients relying only on palliative care to ameliorate symptoms. For CLN7, first experimental treatment approaches are being evaluated for safety and efficacy in pre-clinical and ongoing clinical studies. These include antisense oligonucleotide [6], and a pharmacological treatment with MK2206 mycophenolate, an Akt inhibitor, that seems to enhance in vitro the cellular clearance of ceroid lipopigment deposits in a TFEB-dependent manner [7]. Also, there is a currently ongoing phase 1 open-label clinical trial which recently started the recruitment of patients, based on a single-administration of the gene therapy agent AAV9/CLN7, that will be administered intrathecally into the lumbar spinal cord region of paediatric CLN7 patients (Clinicaltrials.gov ID: NCT04737460).
In this scenario, future therapeutic strategies for all types of BD will need to target beyond the brain and the eye, and to address the likely existence of functionally distinct transcript variants. Hence, the systemic administration of small molecule therapeutics could lead or complement ERT and gene therapy approaches by treating both common and disease-specific effects. In particular, the repurposing of previously approved drugs, rather than an ex-novo testing of novel compounds, represents a recognized and rapid translational opportunity for incurable rare genetic diseases.
By integrating cell-based phenotypic screening and repurposing FDA-approved drugs, we have recently identified tamoxifen (Figure 1), a drug that showed activity ameliorating in vitro and in vivo pathological hallmarks of two different BDs, CLN3 and CLN7 [8]. We found that tamoxifen significantly reduces the intracellular pathological accumulation of Gb3 and the subunit c of the Mitochondrial ATP Synthase (SCMAS) in CLN3 and CLN7 cellular models. Importantly, despite its activity as a Selective Estrogen Receptor Modulator (SERM), we found that tamoxifen is able to ameliorate BD phenotypes through a mechanism that is independent of Estrogen Receptors (ERs), but involves activation of the Transcription Factor EB (TFEB), a master gene of global lysosomal function and autophagy [9]. We found that tamoxifen-mediated induction of TFEB was triggered by lysosomotropic-mediated inhibition of mTORC1. Furthermore, in vivo administration of tamoxifen significantly rescued Cln7Δex2 mutant mice from brain cortex Gb3 accumulation, reducing SCMAS storage, hindlimb clasping, and motor discoordination.
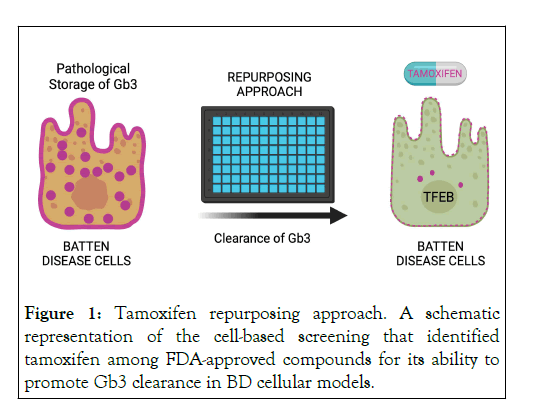
Figure 1: Tamoxifen repurposing approach. A schematic representation of the cell-based screening that identified tamoxifen among FDA-approved compounds for its ability to promote Gb3 clearance in BD cellular models.
Importantly, our data strongly suggest that tamoxifen may be a suitable drug to treat most subtypes of NCLs rather than be specific toward a single disease form, thus widening the plethora of patients that could potentially be eligible for this therapeutic route. In this review, we discuss important clinical aspects that need to be considered to move forward with tamoxifen as a suitable drug for the treatment of multiple BDs.
Tamoxifen clinical aspects
Tamoxifen citrate (hereafter called tamoxifen) is a nonsteroidal SERM, that belongs to the class of organic compounds containing a 1,2-diphenylethylene moiety, known as stilbenes. Tamoxifen is a readily available EMA-and FDA-approved compound, indicated for the treatment of mainly breast cancer in a variety of settings, especially for patients with ER-positive tumors. FDA-approved indications include (i) treatment of breast cancer in both female and male patients [10], (ii) adjuvant treatment of breast cancer in patients who completed a primary treatment with surgery and radiation [11], (iii) treatment of ductal carcinoma in situ (non-invasive breast cancer) in female patients after surgery and radiation to reduce the risk of invasive breast cancer [12], and (iv) breast cancer risk reduction in certain patients with overt predisposition [13]. Tamoxifen also has many off-labelled uses, alone or in combination with other drugs, as shown in Table 1.
| Indications | References |
|---|---|
| Treatment of progressive or recurrent desmoid tumors in combination with sulindac | Hansmann et al., 2004 |
| Treatment of endometrioid histologies that are recurrent, metastatic, or at high-risk | Fiorica et al., 2004, Thigpen et al., 2001 |
| Treatment of primary or secondary gynecomastia along with breast pain associated with it | Boccardo et al., 2005 |
| Induction of ovulation in the treatment of infertility | Steiner et al., 2005 |
| Treatment of oligospermia in combination with testosterone | Adamopoulos et al., 2003 |
| Prophylaxis of coronary arteriosclerosis in men with a triple vessel | Clarke et al., 2001 |
| Treatment of advanced or recurrent ovarian cancer | Hatch et al., 1991 |
| Treatment of bladder cancer | Dellagrammaticas et al., 2001 |
| Treatment of lung cancer in addition to initial chemotherapy treatment | Yang et al., 1999 |
| Treatment of precocious puberty due to McCune-Albright syndrome in females | Eugster et al., 2003 |
| Treatment of metastatic malignant melanoma | Beguerie et al., 2010 |
| Treatment of benign mammary dysplasia | Ricciardi and Ianniruberto, 1979 |
| Treatment of bone metastasis | Spooner and Evans, 1979 |
| Treatment of carcinoid tumor | Moertel et al., 1984 |
| Treatment of cutaneous polyarteritis nodosa | Cvancara et al., 1998 |
| Treatment of hypertrophy of uterus | Magos, 1990 |
| Treatment of meningioma | Markwalder et al., 1985 |
| Treatment of primary breast pain, premenstrual mastodynia, or breast pain that originated from liver cirrhosis | Fentiman et al., 1988, Li et al., 2000, Serels and Melman, 1998 |
| Prophylaxis of postmenopausal osteoporosis | Kristensen et al., 1994 |
| Improvement of length and quality of life in patients with retinoblastoma in addition to treatment protocols | Taçyildiz et al., 2003 |
| Treatment of Riedel's thyroiditis | De et al., 2001 |
| Treatment of solid tumor secondary malignant neoplasms | Lissoni et al., 1996 |
Note: Adapted from Farrar and Jacobs, 2021
Table 1: Non-FDA-approved indications (off-label).
Pharmacokinetics
Human pharmacokinetics of tamoxifen has been extensively reviewed by Morello, et al. [14]. Briefly, upon oral administration tamoxifen is rapidly and extensively absorbed from the gastrointestinal tract [14], peak levels occur after 3 to 7 hours, and steady state is reached typically after 3 to 4 weeks. In terms of distribution, tamoxifen is a highly lipophilic agent, resulting in extensive plasma protein binding (especially to albumin). A human study of tamoxifen distribution showed high concentrations of tamoxifen in liver, lung, pancreas, brain, ovaries and breast tissue [15]. Tamoxifen is metabolized in the liver by the cytochrome P450 enzymes (primarily by CYP3A4, CYP2C9, but also CYP2D6 is involved) into its active metabolites (i.e., endoxifen and afimoxifene) [16]. Tamoxifen is then excreted in bile and mainly eliminated in faeces (only small amounts are found in urine), and has a long elimination half-life of typically 5 to 7 days [16], which is attributed to its high plasma protein binding as well as to enterohepatic recirculation.
Pharmacodynamics
As a nonsteroidal SERM, in tumour cells and other tissue targets, tamoxifen binds to ER in a competitive manner with its endogenous agonist, namely estrogen, which results in the formation of a nuclear complex that reduces DNA synthesis and suppresses estrogen effects. Such anti-estrogenic activity allows tamoxifen to inhibit growth by blocking cells in the G0-G1 phases of the cell cycle. However, depending on the tissue, it can have mixed estrogenic (i.e., uterus and liver) and anti-estrogenic (i.e., breast tissue) activity. As ER agonist, tamoxifen has been associated to increasing incidence of uterine malignancies [17,18], whereas in bone tissue it prevents bone loss, namely osteoporosis, by mimicking the effects of estrogen in osteoclasts [19]. Additionally, it has been described that tamoxifen can promote apoptosis in both ER-positive and ER-negative tumours through the activation of caspases [20-22]. Tamoxifen is considered a long-acting medication since its active metabolite, endoxifen, has a half-life of about 2 weeks. However, it has a narrow therapeutic index, though, as greater doses can cause seizures or difficulties breathing.
Dose-response studies and main non-oncologic clinical studies
Tamoxifen has been tested in a number of clinical trials as a potential therapeutic treatment for non-oncologic conditions (Table 2). For example, a double-blind randomised clinical trial in another neurodegenerative disease, Amyotrophic Lateral Sclerosis (ALS), has tested tamoxifen in 18 patients [23]. Participants were followed up at 1, 3, 6, and 12 months. In this case, the primary endpoint was patient survival or dependence on mechanical ventilation, while secondary endpoints were decline of the revised ALS Functional Rating Scale (ALSFRS-R) score and pulmonary function measured by Forced Vital Capacity (FVC). Results showed that tamoxifen exerted only a modest effect on attenuation of progression for 6 months in this small trial, therefore additional larger scale studies should be necessary to investigate whether, in view of our recent findings in BD, enhancing autophagy through TFEB activation can attenuate ALS progression.
| Clinicaltrials.gov ID | Title |
|---|---|
| NCT02166944 | Tamoxifen treatment in patients with motor neuron disease |
| NCT02835079 | Treatment effect of tamoxifen on patients with Duchenne's Muscular Dystrophy (DMD) |
| NCT00206544 | Anti-Estrogens-A potential treatment for bipolar affective disorder in women? |
| NCT00026585 | Examination of tamoxifen in acute mania in patients with bipolar I disorder |
| NCT03354039 | Tamoxifen in duchenne muscular dystrophy |
| NCT00784940 | ATAC-Bone density sub-protocol |
| NCT00411203 | Tamoxifen in treatment of acute mania |
| NCT00687102 | Cognition in the study of tamoxifen and raloxifene |
| NCT00214110 | Tamoxifen therapy in Amyotrophic Lateral Sclerosis (ALS) |
| NCT01257581 | Safety and efficacy study of creatine and tamoxifen in volunteers With Amyotrophic Lateral Sclerosis (ALS) |
Table 2: Relevant non-oncologic clinical trials.
In addition, the use of tamoxifen in Duchenne Muscular Dystrophy (DMD) children has shown encouraging results in a completed phase 1 trial, in terms of a protective effect on dystrophic muscle from contraction-induced damage, and, importantly, with no adverse effects [24]. Also, tamoxifen demonstrates promising effects in psychiatric disorders, like bipolar disorder, where its therapeutic action may be independent of interaction with ERs through the inhibition of the Protein Kinase C (PKC), a known molecular target in these disorders [25].
Safety
Tamoxifen's safety for paediatric use in BD remains to be fully established. However, certain clinical studies have demonstrated the safety and effectiveness of tamoxifen in young patients. For instance, a patient who took tamoxifen (10 mg TID) for over 3 years was able to successfully delay the premature puberty associated with McCune-Albright Syndrome (MAS) [26]. Additionally, it was discovered that tamoxifen treatment (20 mg QD) for 6 months in adolescents with pubertal gynecomastia was a safe, well-tolerated, and efficient treatment option, as an alternative to other medical treatments [27]. However, prospective and controlled clinical research is required to examine the effectiveness and safety of tamoxifen in young patients affected by lysosomal storage diseases like BD.
The anti-inflammatory and anti-fibrotic properties of tamoxifen led to promising results in DMD patients [24]. The Israeli team has shown that a 6-month compassionate treatment with tamoxifen in 3 boys with DMD improved their condition. Another trial with tamoxifen on DMD was conducted on (i) 79 boys with DMD (ages 6.5 to 12) with the ability to walk and under stable treatment with glucocorticoids, and (ii) on a second group of up to 20 DMD boys (ages 10 to 16), but unable to walk and not under glucocorticoid treatment. Participants were randomly assigned to 20 mg of tamoxifen or placebo QD for 48 weeks. Preliminary data from the trial and its follow-up Open Label Extension (OLE) study confirmed that tamoxifen was safe and generally well tolerated [28]. In addition, a study in a mouse model of muscular dystrophy showed that tamoxifen and raloxifene, also a breast cancer treatment, improved cardiac, respiratory, and skeletal muscle functions, and increased bone density [29]. Overall, adverse effects in these populations have been rare, and tamoxifen seems to have an excellent safety profile. Together with our data, therefore, tamoxifen might represent a novel small molecule therapeutic for at least two types of BD, namely CLN3 and CLN7 diseases.
TFEB activation as strategy to tackle Lysosomal Storage Diseases (LSDs) and neurodegeneration
Lysosomal biogenesis and autophagy are transcriptionally regulated by a gene network and by its master gene TFEB, a member of the basic Helix-Loop-Helix Leucine Zipper (bHLHZip) transcription factors [9,30,31]. TFEB activity responds to environmental cues and is regulated by mTORC1-mediated phosphorylation, which occurs on the lysosomal surface [32]. By siRNA-mediated HCL screening, we discovered that calcineurin, a Ca2+-modulated phosphatase, de-phosphorylates TFEB, thus promoting its nuclear translocation. Interestingly, we also found that calcineurin is activated by a lysosomal calcium signalling mechanism mediated by the lysosomal calcium channel Mucolipin 1 (MCOLN1) [33]. Together these results revealed, for the first time, the presence of lysosome-to-nucleus signaling mechanisms and changed the view of the lysosome from a “suicide bag” to a dynamic organelle that responds to environmental cues. The identification of a global transcriptional regulation of lysosomal function was exploited, first by us and then by other groups, to boost lysosomal function in mouse models of a variety of disease conditions [34-38]. We found that the overexpression of TFEB on cellular models of LSDs promotes the clearance of pathologic lysosomal storage. Also, we found that TFEB induces clearance through the activation of lysosomal exocytosis, by inducing both the predocking of lysosomes close to the plasma membrane and the release of lysosomal calcium to promote lysosomal fusion. Viralmediated TFEB gene transfer resulted in the clearance of accumulating substrates in cells and tissues from mouse models of several types of LSDs [34]. Therefore, the possibility of modulating lysosomal function by acting on TFEB network may lead to a novel therapeutic strategy with potential applicability to more that 50 LSDs as well as more common neurodegenerative diseases. An obvious therapeutic application of these discoveries is the identification of small molecules to activate TFEB pathway in disease conditions. In addition to tamoxifen [8], we have identified and characterized various molecules that regulate TFEB and reduce storage in LSDs, such as the natural product genistein [39,40], and more recently the CNS drug fluoxetine [41].
Mechanisms of action of tamoxifen
Although designed to be a specific ER antagonist, tamoxifen presents many off-target activities [42], including the inhibition of PKC, Calmodulin, and P-Glycoproteins [43]. Thus, in recent years there has been increasing interest within the literature for potential novel “off-target” effects of tamoxifen and its metabolites independent of their anti-ER mechanisms [44]. We found that the therapeutic effect on BD is exerted by tamoxifen through an ER-independent mechanism and discovered that it is actually mediated by the activation of TFEB (Figure 2). In addition, observations on the chemical nature of the compound suggest that the effects of tamoxifen on inducing TFEB activation and reducing intracellular Gb3 storage in CLN3 and CLN7 cell models are due to its weak-base property. Mechanistically, we observed that tamoxifen induced TFEB nuclear translocation by specifically impairing mTORC1- mediated phosphorylation of TFEB without affecting mTORC1 activity toward its canonical substrates, such as S6K, 4EBP and ULK1. Giving the recent observations that RagC/D GTPase activity can mediate selective phosphorylation of mTORC1 substrates [45], we postulated that lysosomotropic properties of tamoxifen specifically affect RagC/D activity leading to the dephosphorylation of TFEB.
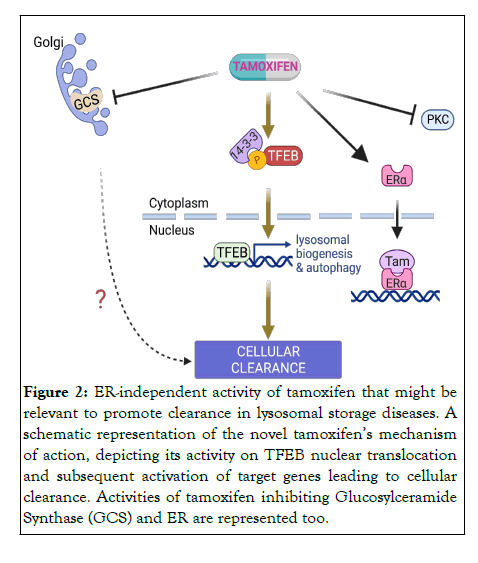
Figure 2: ER-independent activity of tamoxifen that might be relevant to promote clearance in lysosomal storage diseases. A schematic representation of the novel tamoxifenâ??s mechanism of action, depicting its activity on TFEB nuclear translocation and subsequent activation of target genes leading to cellular clearance. Activities of tamoxifen inhibiting Glucosylceramide Synthase (GCS) and ER are represented too.
Whether tamoxifen induces clearance through TFEB-mediated lysosomal exocytosis or other mechanisms still needs to be addressed in future studies. Interestingly, a few reports suggest that tamoxifen can alter glycosphingolipid metabolism in cancer cells [42,46]. Thus, future studies are needed to determine whether the reported effects of tamoxifen on glycosphingolipid regulation may contribute to Gb3 clearance and whether the activation of TFEB is involved (Figure 2).
Tamoxifen-TFEB axis to treat CNS disorders
Tamoxifen already showed a neuroprotective effect in rat ischemia models protecting brain tissue from ischemic injury [47,48]. Similarly, using a canine model of anterior circulation infarction (stroke) to mimic the human clinical condition, Boulos and colleagues were able to consistently show that tamoxifen was effective at significantly improving the canine neurological deficits and reducing the size of the stroke. Interestingly, it has been shown that the activation of TFEB confers neuroprotection in several animal models [49-51]. Consistently, another SERM, raloxifene [52], has shown neuroprotection and immunomodulatory effects in a mouse model of Parkinson’s Disease (PD) [53]. On the other hand, TFEB overexpression studies have suggested it to be a promising therapeutic target to prevent neurodegeneration in various disease models including LSDs and more common neurodegenerative diseases. In PD, AAV-mediated overexpression of TFEB prevented degeneration of dopaminergic neurons in a rat model of α-synuclein induced toxicity [54]. Overexpression of TFEB in both MAPT/Tau and APP-PSEN1/ PS1 mice models ameliorated Tau pathology by reducing biochemical markers such as APP and Aβ production, and rescuing behavioural phenotypes [55,56]. Similarly, TFEB activation cleared huntingtin protein aggregates and reduced neurotoxicity in Huntington's Disease (HD) transgenic mice and cellular models [57].
Together with our discovery that tamoxifen ameliorates pathological hallmarks in BD, these observations support the potential benefits of repurposing tamoxifen (and tamoxifen analogs) to treat LSDs and more common neurodegenerative disorders through the activation of TFEB pathway. Additionally, since most of the approved CNS-penetrant drugs are lysosomotropic, future studies are needed to elucidate whether all these compounds can promote clearance of pathological storage through the activation of TFEB [47-51].
Tamoxifen administration already showed a neuroprotective effect. The identification that tamoxifen, an orally administered compound that crosses the blood-brain barrier and ameliorates two subtypes of neurodegenerative BD in vitro and in vivo, may represent the first small molecule treatment suitable to tackle multiple BD. Future preclinical studies are required to better understand the mechanisms of action of tamoxifen to promote cellular clearance, which might include TFEB activation and glycosphingolipid synthesis inhibition. We envisage that clinical studies may support the translation of these findings into clinics, thus benefiting a number of paediatric patients currently lacking a therapeutic opportunity.
This work was supported by the NCL-Stiftung (NCL Foundation) under the NCL RESEARCH AWARD 2021 program and Fondazione Telethon funding ( # TMDMMFU22TT). All figures were created with Biorender.com.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Soldati C, Montefusco S, Bouché V, Medina DL (2022) Tamoxifen for the Treatment of Batten Disease. J Clin Toxicol. 12:518.
Received: 04-Aug-2022, Manuscript No. JCT-22-18689; Editor assigned: 08-Aug-2022, Pre QC No. JCT-22-18689 (PQ); Reviewed: 22-Aug-2022, QC No. JCT-22-18689; Revised: 29-Aug-2022, Manuscript No. JCT-22-18689 (R); Published: 05-Sep-2022 , DOI: 10.35248/2161-0495.22.12.518
Copyright: © 2022 Soldati C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.