Journal of Chemical Engineering & Process Technology
Open Access
ISSN: 2157-7048
ISSN: 2157-7048
Review Article - (2025)Volume 16, Issue 3
The catalytic conversion of ethanol to Polyethylene Terephthalate (PET) represents a significant advancement in sustainable polymer production, utilizing renewable feedstock’s and pioneering catalytic methodologies. This review examines the intricate multi-step catalytic pathways essential for converting bio-derived ethanol into PET, with a focus on recent innovations in eco-friendly catalyst development and enhancing catalytic efficiency, specifically within fixed bed reactors. The process comprises several key stages: Dehydration of ethanol to ethylene, oxidation of ethylene to Ethylene Glycol (EG) and polymerization of EG with Terephthalic Acid (TPA) to synthesize PET. Each stage presents distinct challenges and opportunities for catalyst improvement and process refinement within the fixed bed reactor configuration. Initially, the dehydration of ethanol to ethylene in a fixed bed reactor requires catalysts with high selectivity, activity and stability. Advanced research has produced promising catalysts like heteropoly acids and zeolites, achieving optimal performance while minimizing energy consumption and by-product formation in the fixed bed setup. The oxidation of ethylene to EG, also within a fixed bed reactor, involves complex reactions. Catalysts such as supported metal oxides and novel nanostructured materials play a crucial role in enhancing reaction kinetics and improving selectivity towards EG, thereby increasing overall process efficiency in the fixed bed environment. The final stage, polymerizing EG with TPA to form PET, necessitates precise control over molecular weight distribution and polymer chain architecture. Innovations in catalyst design, including organometallic complexes and advanced coordination compounds, have led to more efficient polymerization processes in fixed bed reactors, yielding high-quality PET with desirable physicochemical properties.
Ethanol; Polyethylene Terephthalate (PET); Fixed bed reactor; Dehydration of ethanol to ethylene; Oxidation of ethylene to Ethylene Glycol (EG); Polymerization of ethylene glycol with Terephthalic Acid (TPA)
The increasing environmental concerns and depletion of fossil fuel reserves have catalyzed a significant shift towards sustainable and eco-friendly alternatives in various industries. One area of particular interest is the production of polyester fibers, traditionally derived from ethanol. Polyethylene Terephthalate (PET) is a widely used polyester fiber, prominently found in textiles and packaging materials. However, the reliance on petrochemicals for PET production poses substantial environmental challenges [1].
This review explores an innovative approach to producing PET polyester fiber by utilizing ethanol. By converting ethanol, the review aims to provide a sustainable alternative to petro-based PET. The conversion process involves application of noble catalysts to optimize efficiency and yield.
The investigation focuses on understanding the transformation of ethanol into PET. It also examines the catalytic roles of various noble metals in enhancing the conversion process.
Overall, the study underscores the importance sustainable practices in industrial processes, aiming to reduce environmental impact and promote circular economy principles. The findings could pave the way for developing eco-friendly PET, aligning with global efforts to transition towards more sustainable manufacturing practices.
Overall, the study underscores the importance sustainable practices in industrial processes, aiming to reduce environmental impact and promote circular economy principles. The findings could pave the way for developing eco-friendly PET, aligning with global efforts to transition towards more sustainable manufacturing practices.
"Noble catalysts" typically refers to catalysts that are composed of noble metals, such as platinum, palladium, rhodium, ruthenium and iridium. These metals are often used in catalytic converters, chemical processes and various industrial applications due to their ability to accelerate chemical reactions without themselves being consumed in the process.
The term "noble" in this context refers to the metals' resistance to oxidation and corrosion, which allows them to maintain their catalytic activity over long periods. Additionally, their high cost compared to other catalyst materials often makes their use economically significant and requires careful consideration in industrial processes.
Noble catalysts play a crucial role in various fields, including environmental protection (such as reducing emissions from vehicles), petrochemical production, pharmaceutical manufacturing and more.
Several alternatives to noble catalysts exist, each with its own advantages and limitations. Here are some common alternatives:
Base metal catalysts: Base metals such as nickel, copper, iron and cobalt can serve as alternatives to noble metals in various catalytic processes. While base metals are generally less expensive than noble metals, they may exhibit lower catalytic activity, selectivity and durability in some applications. However, advancements in catalyst design and synthesis techniques have led to the development of highly active and selective base metal catalysts for specific reactions [2].
Transition metal oxides: Transition metal oxides, including titanium dioxide, manganese dioxide and cerium oxide, are often used as catalysts in oxidation and redox reactions. These materials can exhibit catalytic activity comparable to or even surpassing that of noble metals in certain applications. Additionally, transition metal oxides are abundant, inexpensive and environmentally friendly, making them attractive alternatives in sustainable catalysis.
Zeolites and Metal-Organic Frameworks (MOFs): Porous materials such as zeolites and MOFs can serve as heterogeneous catalysts by providing high surface areas and well-defined active sites for catalytic reactions. These materials can be tailored to exhibit specific pore structures, surface chemistries and catalytic functionalities, allowing for precise control over reaction selectivity and efficiency. Zeolites and MOFs are particularly useful in gas-phase and liquid-phase reactions, including hydrocarbon conversions, acid-catalyzed reactions and adsorption processes.
Biological catalysts: Enzymes and other biological catalysts offer sustainable and selective alternatives to traditional chemical catalysts. Enzymes are highly efficient and selective catalysts for a wide range of biochemical transformations, including enzymecatalyzed reactions in pharmaceutical synthesis, food processing and bioremediation. However, the application of biological catalysts outside of their native environments may require specialized conditions and engineering approaches to optimize their stability and activity.
Supported catalysts: Supported catalysts consist of active catalytic species dispersed on a solid support material, such as activated carbon, silica or alumina. These catalysts combine the advantages of both the active metal component and the support material, providing high surface areas, improved dispersion of active sites and enhanced stability. Supported catalysts can be tailored for specific applications by varying the composition, morphology and pore structure of the support material and the active phase.
Heterogeneous catalysts: Heterogeneous catalysts are solid-phase catalysts that operate in a different phase (e.g, solid-liquid or solid-gas) from the reactants and products. These catalysts offer advantages such as ease of separation, reuse and scalability compared to homogeneous catalysts. Heterogeneous catalysts encompass a wide range of materials, including metal oxides, zeolites, supported catalysts and nanoparticle catalysts, making them versatile options for various catalytic processes.
Each alternative has its own set of advantages and challenges and the choice of catalyst depends on factors such as the specific reaction requirements, cost considerations, environmental impact and scalability of the process. Advances in catalyst design, synthesis and characterization continue to expand the range of available options and improve the performance and sustainability of catalytic processes [3].
Recent advancement in the conversion of ethanol to pet
The initial step involves the dehydration of ethanol to produce ethylene, a crucial monomer in PET synthesis. This reaction is typically catalyzed by solid acid catalysts. Zeolites such as HZSM-5 and metal oxides like Alumina (Al2O3) and SilicaAlumina (SiO2-Al2O3) have shown high efficiency in this process.
Recent studies have focused on enhancing catalyst stability and activity through modifications such as doping with noble metals (e.g, Pt, Pd) and utilizing hierarchical zeolite structures to increase surface area and pore accessibility. These advancements have led to improved reaction rates and selectivity, reducing energy consumption and by-product formation.
Ethylene is subsequently oxidized to ethylene oxide, which is then hydrated to form Ethylene Glycol (EG), another essential monomer for PET. The oxidation step is efficiently catalyzed by Silver (Ag) catalysts supported on alumina, operating under mild conditions to achieve high selectivity and conversion rates.
The hydration of ethylene oxide to ethylene glycol is typically catalyzed by acidic resins or homogeneous acids like sulfuric acid. Recent advancements have introduced hetero poly acid catalysts and Metal-Organic Frameworks (MOFs), which improve selectivity and reduce by-product formation, making the process more environmentally friendly and economically viable.
Heteropoly acids and their salts: Structures, preparation and catalytic applications in green chemistry [4].
The final step involves the polymerization of ethylene glycol with Terephthalic Acid (TPA) to form PET. TPA is traditionally produced via the catalytic oxidation of para-xylene, using cobaltmanganese-bromide catalysts in acetic acid. Innovations in this area have focused on utilizing CO2-expanded solvents and alternative catalysts to lower reaction temperatures, reduce environmental impact and enhance TPA purity. Additionally, the development of continuous polymerization processes and advanced reactor designs has significantly improved the efficiency and scalability of PET production.
The conversion of ethanol into Polyethylene Terephthalate (PET) involves multiple chemical processes, primarily focusing on the synthesis of the required monomers: Ethylene glycol and terephthalic acid. Utilizing noble metal catalysts in these processes can enhance efficiency and selectivity. Here’s an overview of the steps involved and the potential role of noble catalytic systems.
Step 1: Ethanol to ethylene
The first step in the process is the dehydration of ethanol to produce ethylene:
CH3CH2OH→CH2=CH2+H2O
This reaction is typically catalyzed by alumina or other acidic catalysts, but noble metal catalysts such as Platinum (Pt) or Palladium (Pd) supported on suitable substrates can also be used to enhance the reaction efficiency and selectivity.
The conversion of ethanol to ethylene (dehydration of ethanol) is a well-established process that can be catalyzed by various materials. The choice of the best catalyst depends on factors such as conversion efficiency, selectivity, operating conditions and cost. Here are some of the catalysts commonly used and their characteristics.
Alumina (γ-Al2O3): It has a spinel-like structure with a cubic crystal system. It's a metastable phase of alumina and can convert to the more stable alpha (α-Al2O3) phase upon heating. It is highly effective as a catalyst in the dehydration process due to its acidic properties, which facilitate the removal of a water molecule from ethanol to form ethylene. The high surface area of γ-Al2O3 provides more active sites for the reaction, enhancing the conversion rate. It can withstand the high temperatures required for the reaction without degrading, ensuring consistent catalytic performance.
The reaction typically occurs at temperatures between 300-500°C. γ-Al2O3 can maintain its structure and catalytic properties within this temperature range. The process can be carried out at atmospheric or slightly elevated pressures. γ-Al2O3 catalysts are often prepared by methods like precipitation, sol-gel processes or impregnation with other catalytic materials to enhance performance. The catalyst can be used in fixed-bed reactors where ethanol vapor passes over the catalyst bed or in fluidized-bed reactors where the catalyst is suspended in the gas flow (Figure 1) [5].
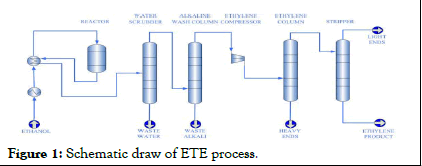
Figure 1: Schematic draw of ETE process.
Zeolites: Zeolites are a group of naturally occurring and synthetic hydrated alum inosilicate minerals known for their unique porous structure. They have a wide range of applications due to their high surface area, uniform pore sizes and ion-exchange properties.
Zeolites have a three-dimensional framework structure composed of SiO4 and AlO4 tetrahedra, linked together by shared oxygen atoms. Zeolites have a network of uniform pores and channels, typically in the range of 3-10 Å in diameter. This porous structure allows zeolites to adsorb molecules of specific sizes, making them excellent molecular sieves.
The extensive internal surface area of zeolites makes them highly effective in adsorption and catalytic applications. Many zeolites are stable at high temperatures, which makes them suitable for use in industrial processes.
Zeolites contain acidic sites that facilitate the dehydration of ethanol. The strength and density of these acidic sites can be tailored by modifying the Si/Al ratio in the zeolite framework.
The uniform pore size of zeolites allows for selective adsorption and reaction of ethanol molecules, which helps in achieving high ethylene selectivity and minimizing side reactions. Zeolites are thermally stable and can withstand the high temperatures typically required for the ethanol-to-ethylene conversion process (Figure 2).

Figure 2: Structure of zeolite.
Noble metal catalysts
Platinum (Pt) supported catalysts: Platinum (Pt) supported catalysts are also explored for the conversion of ethanol to ethylene, although they are less common compared to acidic catalysts like γ-Al2O3 and zeolites. Platinum can facilitate both the dehydration and dehydrogenation reactions, making it versatile in various conditions. Pt facilitates the adsorption and activation of ethanol molecules, promoting the breaking of C-H and O-H bonds [6].
Common supports
Alumina (Al2O3): Provides a high surface area and stability, commonly used to disperse Pt nanoparticles.
Silica (SiO2): Offers thermal stability and an inert surface, suitable for high-temperature reactions.
Zeolites: Combine the acidity of zeolites with the catalytic activity of Pt, potentially enhancing selectivity and activity.
Nanoparticle dispersion: Dispersing Pt as nanoparticles on the support increases the available surface area for the reaction. The support material helps in preventing the agglomeration of Pt nanoparticles, maintaining high catalytic efficiency. Ptsupported catalysts can provide high activity for the ethanol to ethylene conversion, potentially leading to higher yields. Ptsupported catalysts exhibit excellent thermal and chemical stability, making them suitable for high-temperature reactions.
Emerging and alternative catalysts
Transition metal oxides (e.g., ZrO2, TiO2): Transition metal oxides, such as Zirconia (ZrO2) and Titania (TiO2), are widely used as catalysts and catalyst supports in various industrial processes, including the conversion of ethanol to ethylene. Here is an overview of their properties, applications, advantages and drawbacks in this context.
Zirconia (ZrO2)
Crystal structure: ZrO2 exists in three polymorphic forms: Monoclinic, tetragonal and cubic, with the tetragonal and cubic phases being more active catalytically.
Surface acidity and basicity: ZrO2 exhibits both acidic and basic sites, which can facilitate various catalytic reactions.
Thermal stability: ZrO2 is highly stable at elevated temperatures, making it suitable for high-temperature catalytic processes.
Titania (TiO2)
Crystal structure: TiO2 commonly exists in three forms: Anatase, rutile and brookite, with anatase being the most active form catalytically.
Surface properties: TiO2 has high surface acidity and can generate surface oxygen vacancies, which enhance its catalytic activity.
Photocatalytic properties: TiO2 is well-known for its photocatalytic properties, which are useful in environmental applications.
Both ZrO2 and TiO2 can act as catalysts for the dehydration of ethanol to ethylene. They provide active sites for the adsorption and activation of ethanol molecules, facilitating the removal of water and formation of ethylene.
Support for other catalysts: These metal oxides are often used as supports for other active metals (e.g., Pt, Pd) to enhance catalytic performance. The combination of metal oxides with metals can improve the overall catalytic activity, selectivity and stability.
High surface area: Both ZrO2 and TiO2 can be synthesized with high surface areas, providing ample active sites for catalytic reactions. These oxides are stable under harsh reaction conditions, including high temperatures and reactive environments. The acidity and basicity of ZrO2 and TiO2 can be tailored by doping or modifying their surface, making them versatile for various catalytic processes. TiO2, in particular, is non-toxic and widely used in environmental applications, such as air and water purification [7].
Phosphoric acid on Silica (H3PO4/SiO2): Phosphoric acid on Silica (H3PO4/SiO2) is another catalyst used for the conversion of ethanol to ethylene. This catalyst leverages the acidic properties of phosphoric acid supported on the high surface area of silica. Here’s an overview of its use in this reaction.
Phosphoric acid is a strong acid that can provide the necessary acidic sites for the dehydration of ethanol. The acidic sites facilitate the protonation of ethanol, leading to the formation of ethylene and water. The dehydration of ethanol to ethylene occurs on the acidic sites provided by phosphoric acid. Phosphoric acid protonates the ethanol molecule, facilitating the elimination of a water molecule and forming ethylene.
Phosphoric acid is supported on silica, ensuring a high dispersion of the acid and maximizing the exposure of acidic sites to ethanol molecules. The porous nature of silica allows for the efficient diffusion of ethanol and products, improving the overall reaction rate.
H3PO4/SiO2 catalysts offer high selectivity towards ethylene production, minimizing the formation of by-products such as diethyl ether and acetaldehyde.
The strong acidity of phosphoric acid combined with the high surface area of silica results in efficient ethanol conversion at relatively moderate temperatures (typically 300°C-450°C).
The silica support provides thermal stability to the catalyst, maintaining catalytic performance over extended periods.
Silica (SiO2): Silica provides a large surface area for the dispersion of phosphoric acid, enhancing the availability of active sites. Silica is thermally stable, which helps in maintaining the integrity of the catalyst at elevated temperatures required for the reaction [8].
Step 2: Ethylene to ethylene glycol
Ethylene is then oxidized to produce ethylene oxide, which is subsequently hydrolyzed to produce ethylene glycol:
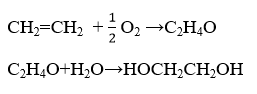
The oxidation of ethylene to ethylene oxide is typically catalyzed by Silver (Ag) catalysts. The subsequent hydrolysis to ethylene glycol does not require noble metals.
The production of ethylene glycol from ethylene typically involves two main steps: The oxidation of ethylene to ethylene oxide, followed by the hydration of ethylene oxide to ethylene glycol. The catalytic methods used in these steps are crucial for achieving high efficiency, selectivity and economic viability.
Ethylene to ethylene oxide
• Catalyst: Silver (Ag) Supported on Alumina (AlO)
• Advantages: High selectivity towards ethylene oxide, established technology with well-known process parameters, good activity and stability under reaction conditions,
• Reaction conditions: Temperature: 200°C-300°C
• Pressure: 1-3 MPa
• Oxygen concentration: Controlled to prevent over-oxidation to CO
• Promoters: Small amounts of other metals (such as Cesium (Cs) or Rhenium (Re)) can be added to the catalyst to enhance selectivity and reduce by-products
Ethylene oxide to ethylene glycol
Non-catalytic hydration (Industrial standard)
Typically carried out using water at high pressures and temperatures without a catalyst.
Reaction conditions:
• Temperature: 200°C
• Pressure: 1-2 MPa
While advanced catalytic methods and materials are being researched, the well-established process using a silver catalyst for ethylene oxidation and non-catalytic hydration remains the most reliable and widely adopted industrial approach. This method provides high selectivity, efficient conversion rates and manageable process conditions, making it the preferred choice for the production of ethylene glycol from ethylene [9].
Step 3: Ethanol to para-xylene
Separately, ethanol can also be converted to para-xylene through a series of reactions involving oligomerization, cyclization and aromatization. Noble metal catalysts, such as those containing platinum or palladium, can facilitate these reactions.
The conversion of ethanol to para-xylene involves a multi-step process including the transformation of ethanol into intermediates like butadiene or mixed xylenes, followed by selective conversion to para-xylene. This can be achieved through catalytic processes involving zeolites and metal catalysts. Here's a detailed look at the best catalytic processes recommended for this transformation.
Ethanol to butadiene
• Catalyst: Bimetallic catalysts (e.g., Ag/MgO or Zn/ZrO)
• Advantages: High selectivity towards butadiene
• Established process with well-understood catalyst behavior
• Reaction conditions: Temperature: 300°C-450°C
• Pressure: Atmospheric to moderate pressures
Butadiene to para-xylene
This involves a series of steps, typically starting with the dimerization of butadiene to form octadiene, which is then cyclized and dehydrogenated to form xylenes, followed by selective production of para-xylene.
• Catalyst: Zeolite-based catalysts (e.g., H-ZSM-5)
• Advantages: High shape selectivity, which enhances the yield of para-xylene
• High thermal stability and resistance to coking
• Reaction conditions: Temperature: 400°C-500°C
• Pressure: Atmospheric to moderate pressures
Direct ethanol to para-xylene via zeolite catalysts
Recent advancements suggest the possibility of converting ethanol directly to para-xylene using specially tailored zeolite catalysts, avoiding the intermediate steps. This is an area of active research and development.
• Recommended catalyst: Phosphorus-modified Zeolite HZSM-5
• Advantages: High selectivity for aromatics, especially paraxylene
• Enhanced catalytic activity and stability due to phosphorus modification
• Reaction mechanism: Ethanol undergoes dehydration to ethylene
• Ethylene oligomerizes to form higher hydrocarbons
• These hydrocarbons cyclize and dehydrogenate to form aromatic compounds, with H-ZSM-5 selectively producing para-xylene
Optimal catalytic process for ethanol to para-xylene
• Catalyst: Phosphorus-modified zeolite H-ZSM-5
• Reaction conditions: Temperature: 400°C-500°C
• Pressure: Atmospheric to moderate pressures
• Feed composition: Pure ethanol feed is preferred for optimal catalyst performance
Advantages of using phosphorus-modified H-ZSM-5
High selectivity: Phosphorus modification enhances the selectivity of H-ZSM-5 towards para-xylene by altering the acidity and pore structure.
Stability: Improved resistance to coking and deactivation, leading to longer catalyst life.
Efficiency: Potential for direct conversion of ethanol to para-xylene reduces process complexity and cost [10].
The direct conversion of ethanol to para-xylene using phosphorus-modified H-ZSM-5 zeolite catalysts represents the best catalytic process currently recommended. This method leverages the high selectivity and stability of modified zeolites, offering an efficient and economically viable route from ethanol to para-xylene. As research progresses, further optimization of this process may lead to even higher efficiencies and broader industrial adoption.
Step 4: Para-xylene to terephthalic acid
Para-xylene is oxidized to terephthalic acid:
equation
This oxidation process can be catalyzed by cobalt-manganesebromide catalysts. While not typically a noble metal process, integrating noble metals can potentially improve the reaction conditions and yield.
The conversion of para-xylene to terephthalic acid is a wellestablished industrial process known as the mid-century process or AMOCO process. This process involves the catalytic oxidation of para-xylene in the liquid phase. Here is a detailed explanation of the catalytic conversion process:
Oxidation of para-xylene to terephthalic acid
The catalyst system used in this process typically consists of:
• Cobalt (Co) and Manganese (Mn) bromides as the primary active catalysts.
• Bromine source (usually hydrogen bromide or sodium bromide) as a promoter to enhance the activity of the Co/Mn system.
Reaction conditions
• Solvent: Acetic acid (serves as the solvent and reacts with oxygen)
• Temperature: 175°C-225°C
• Pressure: 15-30 atm (to maintain acetic acid in the liquid phase and ensure sufficient oxygen solubility)
• Oxygen source: Air or pure oxygen is bubbled through the reaction mixture to provide the necessary oxygen for oxidation
Hydrogenation (for purified terephthalic acid, PTA)
• To obtain high-purity terephthalic acid (often referred to as purified terephthalic acid, PTA), the crude terephthalic acid undergoes a hydrogenation step
• Catalyst: Palladium on Carbon (Pd/C) is commonly used for the hydrogenation of impurities
• Conditions: The hydrogenation is carried out at elevated temperatures (around 250°C) and pressures in the presence of hydrogen gas to reduce impurities like 4-CBA to p-toluic acid, which is more easily removed
Synthesis of PET
Finally, ethylene glycol and terephthalic acid are polymerized to form PET:
equation
The catalytic process for converting para-xylene to terephthalic acid, particularly the mid-century or AMOCO process, is highly efficient and industrially significant. However, it does have several limitations and challenges that can affect its operation and overall efficiency. Here are the major limitations.
Major gaps in observed in the articles
Catalyst deactivation:
• During the dehydration process, carbonaceous deposits (coke) can form on the surface and within the pores of the zeolite. This leads to the blockage of active sites and pores, reducing the catalyst's effectiveness over time.
• Frequent regeneration of the zeolite catalyst is required to remove coke deposits and restore activity, which adds complexity and cost to the process.
• While acidic sites are necessary for the dehydration reaction, excessive acidity can lead to the formation of by-products such as diethyl ether, ethane and aromatics
• Over time and with exposure to high temperatures and steam, the acidic sites of zeolites can degrade, reducing catalytic activity and requiring catalyst replacement or regeneration.
• Although zeolites are thermally stable, the high temperatures (300-500°C) required for the ethanol to ethylene conversion can still lead to structural changes or de alumination in some zeolites, affecting their long-term stability and performance.
• The uniform but relatively small pore size of zeolites can sometimes limit the diffusion of ethanol molecules into the interior active sites, particularly if larger molecules are present. This can reduce the overall efficiency of the catalyst.
• ZrO2 and TiO2 can suffer from deactivation due to coke formation during the ethanol dehydration process, which blocks active sites.
• Compared to other catalysts like zeolites or Pt-based catalysts, ZrO2 and TiO2 may have lower intrinsic catalytic activity for ethanol dehydration.
• Achieving the optimal balance of acidic and basic sites on ZrO2 and TiO2 can be challenging, requiring precise control over synthesis and modification processes.
• Phosphoric acid leaching: Under certain conditions, phosphoric acid may leach from the silica support, reducing the catalyst’s effectiveness.
• Phosphorus deposition: Phosphorus modification can lead to a decrease in the overall acidity of the catalyst, which may reduce its catalytic activity. This reduction in activity can slow down the conversion rates, making the process less efficient.Uniform phosphorus distribution and optimal modification conditions can be challenging.
• Phosphorus-modified catalysts can still be prone to coking (the formation of carbon deposits), which can lead to catalyst deactivation over time. This necessitates regular regeneration or replacement of the catalyst, adding to operational costs.
• The introduction of phosphorus can sometimes alter the selectivity of the catalyst, leading to the formation of unwanted by-products. This can complicate downstream processing and purification steps, reducing overall process efficiency.
• High temperatures required for certain reactions might lead to phosphorus volatilization or other stability problems.
• The use of phosphorus compounds in catalyst preparation and modification can pose environmental and health risks.
• Regenerating phosphorus-modified catalysts can be more complex compared to unmodified versions.
Bromine volatility:
• Bromine, used as a promoter, can volatilize and cause catalyst degradation over time.
By-products and impurities:
• A significant impurity in the crude terephthalic acid, requiring additional purification steps.
• Other aldehydes formed during oxidation can complicate purification and reduce product yield.
• The presence of water, a by-product of the ethanol dehydration reaction, can affect the performance of some zeolites. Water can compete for active sites, reduce the catalyst's acidity and lead to hydrothermal degradation over time.
• The presence of water, a by-product of the dehydration reaction, can impact the stability of the phosphoric acid on the silica support, potentially leading to decreased catalytic performance over time.
Environmental and safety concerns:
• The use of acetic acid as a solvent poses risks of corrosion and requires careful handling to avoid environmental contamination.
• Bromine is toxic and corrosive, necessitating strict safety measures and containment protocols to prevent exposure and environmental release.
• The process operates at high pressures and temperatures, increasing the risk of equipment failure and requiring robust safety systems.
Energy and resource intensity:
• Maintaining the required reaction conditions (temperature and pressure) consumes significant energy, impacting the process's overall sustainability.
• The need for a continuous and controlled supply of oxygen or air increases operational complexity and costs.
Catalyst recovery and recycling
• Loss of catalyst activity over time and during recovery/ recycling processes can lead to increased operational costs.
• Efficient recovery and recycling of the catalyst components (cobalt, manganese and bromine) require sophisticated systems and can be economically challenging. •The need for regular regeneration to remove coke deposits can be complex and requires additional equipment and procedures, increasing operational costs and downtime.
Waste management
• The process generates wastewater and acetic acid by-products, which require effective treatment and management to meet environmental regulations.
• Spent catalyst and solid by-products need proper disposal or recycling, adding to the waste management burden.
High cost of production
• Platinum is an expensive metal, which increases the overall cost of the catalyst and can impact the economic viability of the process. Pt catalysts are susceptible to poisoning by impurities such as sulfur and carbon monoxide, which can lead to catalyst deactivation. The formation of carbonaceous deposits (coke) can also deactivate the catalyst, requiring regeneration or replacement.
• While Pt can be highly active, it may not always be as selective as other catalysts like γ-AlO or zeolites in producing ethylene. Side reactions may produce by-products such as diethyl ether or acetaldehyde.
Scale-up challenges
• Integrating the oxidation process with downstream purification and hydrogenation steps can be complex, particularly in large-scale operations.
• High initial capital investment for reactors, high-pressure equipment and safety systems can be a barrier for new installations.
Purity of feed
• Purity of ethanol can significantly impact catalyst performance; high-purity ethanol is preferred to minimize catalyst fouling.
Strategies to bridge the gap
• Developing strategies to minimize coke formation, such as optimizing reaction conditions and using co-catalysts, can help extend catalyst life.
• Balancing the acidity is crucial to maintain high selectivity towards ethylene.
• Engineering zeolites with optimized pore sizes and acidity can enhance selectivity and reduce the formation of by-products.
• Implementing advanced regeneration techniques, such as oxidative regeneration or steam treatment, can improve the efficiency and lifespan of the zeolite catalyst.
• Combining zeolites with other catalytic materials (e.g., metal oxides) can enhance performance and stability while mitigating some of the drawbacks associated with pure zeolite catalysts.
• Doping ZrO or TiO with other elements (e.g., Ce, La) can enhance their catalytic properties by increasing surface acidity or creating more oxygen vacancies.
• Combining ZrO or TiO with other catalytic materials (e.g., zeolites, noble metals) can improve overall performance and reduce the likelihood of deactivation.
• Careful control of reaction temperature, pressure and feed composition can minimize coke formation and prolong catalyst life.
• Fine-tuning the reaction temperature, pressure and ethanol feed rate can minimize coke formation and extend catalyst life.
• Modifying the preparation method of the HPO/SiO catalyst to enhance the stability of phosphoric acid on the silica support can improve performance.
• Adding promoters or co-catalysts that enhance coke resistance and improve the dispersion of phosphoric acid can enhance the overall catalytic activity and longevity.
•Fine-tuning the reaction temperature, pressure and space velocity can help enhance the selectivity and yield of ethylene. Combining Pt with other metals (e.g., Sn, Zn) can enhance catalytic performance and selectivity while potentially lowering costs. Modifying the support material (e.g., introducing acidic sites) can improve the overall performance of Pt-supported catalysts in ethanol dehydration.
• The noble metals like Pt and Pd offer high performance, their cost can be prohibitive. Zeolite-based catalysts, particularly HZSM-5, provide an excellent balance of activity, selectivity and cost, making them a preferred choice for the ethanol to ethylene conversion process.
Future work to be done
Future work in the production of PET (Polyethylene Terephthalate) from ethanol could focus on several key areas to enhance efficiency, sustainability and economic viability:
Development of novel catalysts: Research could focus on developing new catalysts or modifying existing catalysts to improve the direct conversion of ethanol to Terephthalic Acid (TPA), a key precursor in PET production. Catalysts that enhance selectivity, reduce by-products and operate under milder conditions (lower temperature and pressure) could be explored. Phosphoric acid on silica is historically significant due to its high activity and selectivity, but due to handling and environmental concerns, modern processes often favor other options.
Integration of advanced reaction technologies: Investigating advanced reaction technologies such as membrane reactors, enzyme catalysis or electrochemical methods could offer more efficient and sustainable pathways for TPA production from ethanol. These technologies may reduce energy consumption, minimize waste generation and improve process economics.
Enhanced process integration: Optimizing process integration and intensification strategies to streamline the ethanol-to-TPA conversion process. This could involve coupling reaction steps, maximizing heat and mass transfer efficiencies and integrating renewable energy sources for process heating and electricity.
Focus on sustainability: Increasing the sustainability of PET production by utilizing bio-based ethanol from renewable biomass sources rather than fossil fuels. This shift would reduce the carbon footprint of PET production and align with global sustainability goals.
Catalyst and process scale-up: Scaling up promising catalysts and processes from laboratory-scale to industrial-scale production. Addressing scalability challenges, such as catalyst stability, reactor design and cost-effectiveness, is crucial for industrial adoption.
Process modeling and simulation: Utilizing advanced modeling and simulation techniques to predict and optimize the performance of ethanol-to-TPA conversion processes. This approach can help in understanding reaction kinetics, optimizing operating conditions and reducing experimental costs.
Circular economy approaches: Implementing circular economy principles by exploring ways to recycle and reuse by-products and waste streams generated during the ethanol-to-TPA conversion process. This includes developing technologies for efficient recovery and recycling of catalysts and other materials.
The noble catalytic conversion of ethanol into PET exemplifies the synergy between advanced catalysis and sustainable industrial practices. Ongoing research and development in catalyst optimization, process integration and environmental impact assessment are crucial to realizing the full potential of this technology. By leveraging renewable feed stocks and innovative catalytic methods, the production of PET from ethanol can contribute significantly to the circular economy and the global transition towards sustainable materials.
While the catalytic process for converting para-xylene to terephthalic acid is efficient and well-established, it faces limitations related to catalyst deactivation, by-products, environmental and safety concerns, energy and resource intensity, product purity, catalyst recovery, waste management and scale-up challenges. Addressing these limitations requires ongoing research, process optimization and innovation in catalyst design and reaction engineering.
By integrating noble metal catalysts at various stages, the overall process can become more efficient, with potential improvements in reaction rates, selectivity and product purity. However, the economic viability and catalyst regeneration/recycling also need to be considered for industrial-scale applications.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Tasew D, Tefera N (2025) Synthesis of Polyethylene Terephthalate (PET) Polyster Fiber from Bio Based Ethanol in a Noble Catalytic Conversion Process in a Fixed Bed Reactor. J Chem Eng Process Technol. 16:542.
Received: 11-Jul-2024, Manuscript No. jcept-24-32867; Editor assigned: 16-Jul-2024, Pre QC No. jcept-24-32867 (PQ); Reviewed: 30-Jul-2024, QC No. jcept-24-32867; Revised: 13-Jun-2025, Manuscript No. jcept-24-32867 (R); Published: 20-Jun-2025 , DOI: 10.35248/2157-7048.25.16.542
Copyright: © 2025 Tasew D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.