Organic Chemistry: Current Research
Open Access
ISSN: 2161-0401
ISSN: 2161-0401
Research Article - (2015) Volume 4, Issue 4
Four new Macrocyclic Hydrazone Schiff bases were synthesized by condensation of intermediate compounds: 1,6- bis (2-formyl-phenyl) hexane, 1,6-bis (2-acetyl-phenyl)hexane(V), α,α’-bis(2-carboxyaldehyde phenoxy) xylene(VI), and 1,7-bis (2-formyl-phenyl)-1,4,7-trioxaheptane(VII) with 1,3-Dithio-carbohydrazide(III) in the molar ratio (2:2) in DMF. Identification of these macrocyclic Schiff bases ligands (VIII, IX, X, XI). The Schiff bases were checked by different spectral technique (LC-MS, 1H-NMR, IR, elemental analyses). These compounds were tested to determine their ability to inhibit corrosion of mild steel in 1M H2SO4 by Electrochemical measurements. The new Macrocyclic Hydrazone Schiff Bases were studied for antibacterial activities against Gram positive (Bacillus subtilis and Staphylococcus aureus) and Gram negative (Salmonella typhi and Escherichia coli). The compound ligands exhibited a variable activity of inhibition on the growth of the bacteria.
Keywords: Macrocyclic hydrazone; 1,3-Dithio-carbohydrazide; Anticorrosion; Antibacterial activity
Schiff bases are widely studied and used in the fields of organic synthesis and metal ion complex [1,2] for a number of reasons: their physiological and pharmacological activities [3-5] their use in ion selective electrodes [6-11], in the determination of heavy metals ions in environmental samples [12], in the extraction of metals ions [13,14] and their many catalytic applications e.g., for epoxidation of olefins, alkene cyclopropanation [15,16] trimethylsilylcyanation of ketones [17] asymmetric oxidation of methyl phenyl sulfide enantioselective epoxidation of silylenol [18] and ring-opening Polymerization of lactide [19]. Hydrazones are special group of compounds in the Schiff bases family. They are characterized by the presence of (C=NN=C). The presence of two inter-linked nitrogen atoms was separated from imines, oximes etc. Hydrazone Schiff bases of acyl, aroyl and heteroacroyl compounds have additional donor sites like C=O. The additional donor sites make them more flexible and versatile. This versatility has made hydrazones good polydentate chelating agents that can form a variety of complexes with various transition and inner transition metals and have attracted the attention of many researchers. Various hydrazones are obtained depending on the experimental conditions; which have application as biologically active compounds [20] and as analytical reagents [21]. As biologically active compounds, hydrazones find applications in the treatment of diseases such asanti-tumor [22], tuberculosis [22], leprosy and mental disorder [23]. For Tuberculostatic activity is attributed to the formation of stable chelates with transition metals present in the cell. Thus many vital enzymatic reactions catalyzed by these transition metals cannot take place in the presence of hydrazones [24,25]. Hydrazones also act as herbicides, insecticides, nematicides, rodenticides, plant growth regulators.
Reagents and apparatus
All the chemicals used were of Analytical grade and procured from Sigma-Aldrich and Fluka. Metal salts were purchased from E. Merckand were used as received. The elements C, H, and N were analyzed ona Carlo-Erba 1106 elemental analyzer. The IR spectra were recorded on Jusco 300 instrument in KBr pellets. 1H NMR spectra of ligands in CDCl3 solution were recorded on a Bruker DT-400 MHz spectrometer and chemical shifts are reported in ppm. Mass spectra were recorded used a KRATOS MS50TC spectrometer. AA 929 Unicam Spectrometer was used for FAAS measurements with an airacetylene flame. A pH meter (Metrohm -691 pH Meter) was also used. All extraction experiments were performed by using a mechanical flask agitator in 50 cm3 stoppered glass flasks, M.P Apparatus Digital (32- 300°C). Potantiostat - galvanostat from Amel instruments were used for corrosion activity.
Synthesis of dimethyl isophthalate (I)
Isophthalic acid (1.66 gm, 0.1 mmol) in super dry methanol (60 mL) containing 2-3 drop wise of concentrated H2SO4(AR) was refluxed till it dissolved. Then, there action mixture was poured onto ice cold water, immediately a solid started separating from the clear solution. To this a solution of sodium bicarbonate was added till the effervescence seized. The ester thus obtained was filtered and washed with water for several times (MP: 64 - 67°C) [26].
Synthesis of dihydrazide of isophthalic acid (II)
A mixture of dimethyl ester of isophthalic acid (2.22 gm) and hydrazine hydrate (98%, 2 cc) in methanol was refluxed for 4-5 hours. The reaction mixture was allowed to cool to room temperature then, the cooled solution was poured on to ice cold water. The dihydrazide of isophthalic acid thus obtained was filtered and recrystallized from ethanol [27]. Yield: (85%), MP: 241°C, Empirical formula: (C8H10N4O2), M. Wt: (194 gm) (Scheme 1).
Synthesis of 1,3-Dithiocarbohydrazide (III)
To a suspension of 1,3-Dicarbohydrazide (2.37 g, 12.2 mmol) in 20 mL of THF Lowes sons reagent (4.95 g, 12.2 mmol) was added at room temperature, and the mixture was refluxed up to the formation of homogeneous solution (for 2 h). After removal of the solvent the residue was solved in 15 mL CH2Cl2 and allowed to solidify. The crystals were dried and recrystallized from acetone. 1,3-Dithiocarbohydrazide was obtained, yellow crystals [28], Yield: (80%), M.P: 262ºC, Empirical formula: (C8H10N4S2), M. Wt: (227 gm) (Scheme 2).
Synthesis of 1,6-bis(2-formylphenyl)hexane (IV)
To a stirred solution of salicylaldehyde (24.4 gm, 0.2 mol) and K2CO3 (13.8 gm, 0.1 mol) in DMF (100 mL), was added drop wise 1,6-dibromo hexane (12.2 gm, 0.01 mol) in DMF (40 mL). The reaction was continued for 4 hours at 150-155ºC and then for 4 hours at room temperature. Then, 200 mL distilled water was added and the mixture was kept in refrigerator. After 1 hour, the precipitate was filtered and washed with 500 ml water. It was dried in air and recrystallized from EtOH and filtered under vacuum [29]. Yield: 85%, M.P: 75°C, Empirical formula: (C20H22O4), M. Wt : (326 gm) (Scheme 3).
Synthesis of 1,6-bis (2-acetylphenyl)hexane (V)
To a stirred solution of 2- hydroxyl acetophenone (13.6 g, 0.1 mol) and K2CO3 (6.9 g, 0.05 mol) in DMF (50 mL), was added drop wise 1,6-dibromo hexane (6.1 g, 0.05 mol) in DMF (20 mL). The reaction was continued for 4 h at 150-155°C and then for 4 h at room temperature. Then, 100 mL distilled water was added and the mixture was kept in refrigerator. After 1 h, the precipitate was filtered and washed with 250 ml water. It was dried in air and recrystallized from EtOH and filtered under vacuum [29]. Yield: 80%, M. P: 122°C, Empirical formula: (C22H26O4), M. Wt: (354 g) (Scheme 4).
Synthesis of α,α’-bis(2-carboxyaldehyde phenoxy)xylene (VI)
To a stirred solution of salicylaldehyde (24.4 g, 0.2 mol) and K2CO3 (13.8 g, 0.1 mol) in DMF (100 mL), was added drop wise α,α’-Dichlorp- xylene (17.4 g, 0.1 mol) in DMF (40 mL). The reaction was continued for 4 h at 150-155°C and then for 4 h at room temperature. Then, 200 mL distilled water was added and the mixture was kept in refrigerator. After 1 h, the precipitate was filtered and washed with 500 mL water. It was dried in air and recrystalized from EtOH and filtered under vacuum [29]. Yield: 75%, M. P: 107°C, Empirical formula: (C22H18O4), M. Wt: (346 g) (Scheme 5).
Synthesis of 1,7-bis (2-formylphenyl)-1,4,7-trioxaheptane (VII)
To a stirred solution of salicylaldehyde (2.44 g, 0.02 mol) and K2CO3 (1.38 g, 0.01 mol) in DMF (50 mL), was added drop wise 1-chloro- 2-(2-chloroethoxy)ethane (1.43 g, 0.01 mol) in DMF (20 mL). The reaction was continued for 4 h at 150-155°C and then for 4 h at room temperature. Then, 200 mL distilled water was added and the mixture was kept in refrigerator. After 1 h, the precipitate was filtered and washed with 500 ml water. It was dried in air and recrystalized from EtOH and filtered under vacuum. Yield: 81%, M.P: 75°C, Empirical formula: (C18H18O4), M. Wt: (314 g) (Schemes 6 and 7).
Synthesis of 1,2,8,9-tetraza-4,6; 11,12; 20,21-tribenzo-3,7- dicarbonyl-13, 16, 19- trioxa - cyclodocosane-1,9-diene (VIII)
The macrocyclic Schiff base (VIII) was prepared by drop wise addition of a solution of the 1,3-Dithiocarbohydrazide(III) (0.452 g, 0.002 mol) in DMF (40 mL) to a stirred solution of 1,7-bis(2- formylphenyl) -1,4,7-trioxaheptane (VII) (0.628 g, 0.002 mol) in DMF (60 mL) containing a few drops of concentrated HCl. The reaction mixture was heated to reflux for 5 h, where yellow precipitate was formed after cooling. On cooling, 200 ml distilled water was added and the mixture was kept in a refrigerator. After 2 h, the precipitate was filtered and washed with 200 mL water. The solid obtained was collected and recrystallized from mixture DMF, ethanol (9:1) as yellow crystals. A yellow colored precipitate was washed with water, ethanol, CHCl3 and diethyl ether, respectively. Then dried in air (Scheme 8(. Yield: 60%. M. P: >300°C. Anal. Calc. for C26H24N4O3S2 : C: 61.88, H: 4.79, N: 11.10, S: 12.71. Found: C: 61.99, H: 4.74, N: 11.15, S: 12.65%. Mass spectrum (LCMS): m/z= 504 ([C26H24N4O3S2]). IR (KBr disk): 3167.12-3201.83 cm-1 (CS-NH-), 3008.95-3043.67 cm- 1(C-H), aromatic), 2854.65 cm-1(C-H), aliphatic), 1674.21 cm-1(C=S), 1635.64 cm-1 (C=N), 1593.0 cm-1 (C=C, aromatic), 1300.02 cm-1 (C-O), aromatic) (Figure 1). 1H-NMR(CDCl3 – 400 MHz) δ =12.513 (s,2H, CS-NH-), 11.117 (s,2H,CH=N), 6.943 - 8.903 (m,12H, Ar-H), 2.508- 3.361 (m,8,(-O-CH2CH2-O-)2 (Figure 5).
Synthesis 1,2,8,9-tetraza-4,6; 11,12; 21,22-tribenzo -3,7-dithiocarbonyl -13,20-dioxa-cyclotricosane-1,9-diene (IX)
The macrocyclic Schiff base (IX) was prepared by drop wise addition of a solution of the 1,3-Dithiocarbohydrazide(III) (0.452 g, 0.002 mol) in DMF (40 mL) to a stirred solution of 1,6-bis (2-formylphenyl)hexane (IV) (0.652 g, 0.002 mol) in DMF (60 mL) containing a few drops of concentrated HCl. The reaction mixture was heated to reflux for 5 h, where yellow precipitate was formed after cooling. On cooling, 200 ml distilled water was added and the mixture was kept in a refrigerator. After 2 h, the precipitate was filtered and washed with 200 mL water. The solid obtained was collected and recrystallized from mixture DMF, EtOH (9:1) as yellow crystals. A yellow colored precipitate was washed with water, ethanol, CHCl3 and diethyl ether, respectively. Then dried in air (Scheme 9). Yield: 65%. MP: >300°C. Anal. Calc. for C28H28N4O2S2 : C: 65.09, H: 5.46, N: 10.84, S: 12.41. Found: C: 69.14, H: 5.86, N: 11.02, S: 12.23%, Mass spectrum (LCMS): m/z=516 ([C28H28N4O2S2]). IR (KBr disk): 3236.8 - 3414.3 cm-1 (CS-NH-), 3072.2 cm-1(C-H), aromatic), 2866.0-2939.3 cm-1(C-H), aliphatic), 1637.2 cm-1 (C=S), 1616.8 cm-1 (C=N), 1599.3 cm-1 (C=C, aromatic), 1245.2 cm-1(C-O) (Figure 2). 1H-NMR(CDCl3-400 MHz) δ=12.511 (s,2H, CS-NH-), 8.954 (s,2H,CH=N), 7.121-8.391 (m,12, Ar), 4.147 (s,4H,-O-CH2), 1.873 - 2.223 (m,8H,-CH2CH2CH2CH2) (Figure 6).
Synthesis of 1,2,8,9-tetraza-4,6; 11,12; 21,22-tribenzo -3,7-dithiocarbonyl -13,20-dioxa-10,23-dimethylcyclotricosane- 1,9-diene (X)
The macrocyclic Schiff base (X) was prepared by drop wise addition of a solution of 1,3-Dithiocarbohydrazide(III) (0.452 g, 0.002 mol) in DMF (40 mL) to a stirred solution of 1,6-bis (2-acetylphenyl)hexane (V) (0.708 g, 0.002 mol) in DMF (60 mL) containing a few drops of concentrated HCl. The reaction mixture was heated to reflux for 5 h, where yellow precipitate was formed after cooling. On cooling, 200 ml distilled water was added and the mixture was kept in a refrigerator. After 2 h, the precipitate was filtered and washed with 200 mL water. The solid obtained was collected and recrystallized from mixture DMF, EtOH (9:1) as yellow crystals. A yellow colored precipitate was washed with water, ethanol, CHCl3 and diethyl ether, respectively. Then air dried (Scheme 10). Yield: 80%. MP: >300°C. Anal. Calc. for C30H32N4O2S2: C: 66.15; H, 5.92; N, 10.29; S, 11.77. Found: C:65.99, H:6.08, N:11.20, S:11.86%, Mass spectrum (LCMS): m/z= 544 ([C30H32N4O2S2]). IR (KBr disk): 3224.3-3415.9 cm-1 (CS-NH-), 3066.2 cm-1 (C-H), aromatic), 2866.0-2939.3 cm-1(C-H), aliphatic), 1726.6 cm-1 (C=S), 1651.0 cm-1 (C=N), 1599.0-1578.5 cm-1 (C=C, aromatic), 1267.0 cm-1 (C-O) (Figure 3). 1H-NMR(CDCl3-400 MHz) δ=10.923 (s,2H, CS-NH-), 6.978 - 7.664 (m,12, Ar), 4.126 – 4.211 (s,4H,-O-CH2), 3.942(N=C-CH3), 1.305-2.868 (m,8H,-CH2CH2CH2CH2) (Figure 7).
Synthesis of 1,2,8,9-tetraza-4,6; 11,12;15,18; 21,22-tribenzo -3,7-dithiocarbonyl -13,20-dioxa-cyclotricosane-1,9-diene (XI)
The macrocyclic Schiff base (XI) was prepared by drop wise addition of a solution of the 1,3-Dithiocarbohydrazide(III) (0.452 g, 0.002 mol) in DMF (40 mL) to a stirred solution of α,α’-bis(2- carboxyaldehyde phenoxy) xylene (VI) (0.692 g, 0.002 mol) in DMF (60 mL) containing a few drops of concentrated HCl. The reaction mixture was heated to reflux for 5 h, where yellow precipitate was formed after cooling. On cooling, 200 ml distilled water was added and the mixture was kept in a refrigerator. After 2 h, the precipitate was filtered and washed with 200 mL water. The solid obtained was collected and recrystallized from mixture DMF, EtOH (9:1) as yellow crystals. A yellow colored precipitate was washed with water, ethanol, CHCl3 and diethylether, respectively. Then dried air (Scheme 11). Yield: 75%. MP: >300°C. Anal. Calc. for C30H24N4O2S2: C: 67.14, H: 4.51, N: 10.44, S: 11.95 Found: C: 66.98, H: 4.68, N: 10.56, S: 11.98%, Mass spectrum (LCMS): m/z=536 ([C30H24N4O2S2]). IR (KBr disk): 3414.7-3477.0 cm-1 (CS-NH-), 3072.7 cm-1, 2871.4- 2950.4 cm-1 (C-H), aliphatic), 1663.6 cm-1 (C=S), 1637.6 cm-1 (C=N), 1594.9-1617.5 cm-1 (C=C, aromatic), 1244.3 cm-1 (C-O), aromatic) (Figure 4). 1H-NMR(CDCl3-400 MHz) δ=13.133 (s,2H, CS-NH-), 9.035 (s,2H,CH=N), 76.079-7.923 (m,16H, Ar-H), 3.723-3.982 (s,4H,-O-CH2-), 2.179-3.400 (Solvents organic) (Figure 8).
Anticorrosion activity
Electrochemical measurements were carried out in conventional three-electrode system in CHI 604 instrument (USA) at 303 K. The working electrode (mild steel) has a geometric area of 1 cm2. The saturated calomel and platinum electrodes were used as reference and auxiliary electrodes. Equation (1) shows the calculation of IE from corrosion current [29-33]:
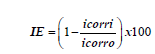 (1)
(1)
Biological activity
The prepared compounds were tested for their antimicrobial activity against four species of bacteria (Bacillus subtilis, Escherichiacoli, Staphylococcus aureus, Salmonella typhi) using filter paper disc method [34]. The screened compounds were dissolved individually in DMSO (dimethyl sulfoxide) in order to make up a solution of 50, 100, and 200 μg/ml concentration for each of these compounds. Filter paper discs (Whatman No.1 filter paper, 5 mm diameter) were saturated with the solution of these compounds. The discs were placed on the surface of solidified Nutrient agar dishes seeded by the tested bacteria. The diameters of inhibition zones (mm) were measured at the end of an incubation period, which was 24 hours at 37ºC for bacteria. Discs are saturated with DMSO are used as solvent control. Ciprofloxacin 100 μg/ml was used as reference substance for bacteria [35].
Synthesis
The prepared macrocyclic hydrazone (VIII, IX, X, XI) were synthesized by condensation of intermediate compounds: 1,6- bis(2-formyl-phenel) hexane,1,6-bis (2-acetyl-phenyl)hexane(V), α,α’-bis(2-carboxyaldehyde phenoxy) xylene(VI), and 1,7-bis (2-formyl-phenyl)-1,4,7-trioxaheptane(VII) with 1,3-Dithiocarbohydrazide( III) in the molar ratio (2:2) in DMF. The reactions proceeded smoothly, producing the corresponding Schiff bases ligands in good yield. The ligands are soluble in common organic solvent but insoluble in water. The structures of the ligands were elucidated by elemental analyses, MS, FTIR, electronic absorption, and 1H- NMR spectra, which help in elucidating their empirical formula (Table 1).
| Schiff base | Color | M.Wt | Melting point (°C) | Yield % | Crystallization |
|---|---|---|---|---|---|
| Solvent | |||||
| VIII | yellow | 504 | >300 | 60 | DMF, EtOH (9:1) |
| IX | yellow | 516 | >300 | 65 | DMF, EtOH (9:1) |
| X | yellow | 544 | >300 | 80 | DMF, EtOH (9:1) |
| XI | yellow | 536 | >300 | 75 | DMF, EtOH (9:1) |
Table 1: Color, molecular weight and melting point of macrocyclic hydrazone (VIII, IX, X, XI).
Elemental analyses of macrocyclic hydrazone (VIII, IX, X, XI)
The results of elemental analyses macrocyclic hydrazone (VIII, IX, X, XI), as shown in Table 2, are in good agreement with those required by the proposed formulae.
| Schiff bases | Elemental analysisCalculated (Found %) | |||
|---|---|---|---|---|
| C | H | N | S | |
| VIII | 61.88 (61.99) | 4.79 (4.74) | 11.10 (11.15) | 12.71 (12.65) |
| IX | 65.09 (64.99) | 5.46 (5.52) | 10.84 (11.02) | 12.41 (12.23) |
| X | 66.15 (65.98) | 5.92 (6.01) | 10.29 (10.16) | 11.77 (11.86) |
| XI | 67.14 (66.98) | 4.51 (4.68) | 10.44 (10.56) | 11.95 (11.98) |
Table 2: Elemental analysis data of macrocyclic hydrazone (VIII, IX, X, XI).
IR spectra analysis
Compound (VIII): A strong band at 1635.64 and 1674.21 cm-1 in the IR spectrum of the Schiff base (Figure 1) are assigned to υ(C=N) of azomethine and thiocarbonyl υ(C=S) vibrations, respectively. An intense band at 3201.83-3167.12 cm-1 is due to the -NH- vibrations of the hydrazine group The band in the spectra at 1593.0 cm-1 is due to (C=C) of aromatic rings. While the band at 2854.65 cm-1 are attributed to (C-H aliph). Also, the band at 3043.67-3008.95 cm-1 are attributed to (C-H ar) [36-40].
Compound (IX): A strong band at 1616.8 and 1637.2 cm-1 in the IR spectrum of the Schiff base (Figure 2) are assigned to υ(C=N) of azomethine and thiocarbonyl υ(C=S) vibrations, respectively. An intense band at 3414.3-3236.8 cm-1 is due to the -NH- vibrations of the hydrazine group The band in the spectra at 1599.3 cm-1 is due to (C=C) of aromatic rings. While the band at 2939.30-2868 cm-1 are attributed to (C-H aliph). Also, the band at 3072.2 cm-1 are attributed to (C-H ar) [36-40].
Compound (X): A strong band at 1651 and 1726.6 cm-1 in the IR spectrum of the Schiff base (Figure 3) are assigned to υ(C=N) of azomethine and thiocarbonyl υ(C=S) vibrations, respectively. An intense band at 3415.9-3224.3 cm-1 is due to the -NH- vibrations of the hydrazine group The band in the spectra at 1599.0 -1578.5 cm-1 is due to (C=C) of aromatic rings. While the band at 2939.3-2866.0 cm-1 are attributed to (C-H aliph). Also, the band at 3066.2 cm-1 are attributed to (C-H ar) [36-40].
Compound (XI): A strong band at 1637.6 and 1663.6 cm-1 in the IR spectrum of the Schiff base (Figure 4) are assigned to υ(C=N) of azomethine and thiocarbonyl υ(C=S) vibrations, respectively. An intense band at 3477.0-3414.7 cm-1 is due to the -NH- vibrations of the hydrazine group. The band in the spectra at 1617.5-1594.9 cm-1 is due to (C=C) of aromatic rings. While the band at 2950.4-2871.4 cm-1 are attributed to (C-H aliph). Also, the band at 3072.7 cm-1 are attributed to (C-H ar) [36-40].
However, in the IR spectra of Schiff bases this bands (C=S) disappears and a new vibration bands for azomethine (-HC=N-). Indicating that complete condensation takes place. All IR spectral data of the synthesized compounds showed in the Table 3 [41-42].
| Schiff bases | v(C-O) | v(C=C) | v(C=N) | v(C=S) | C-H aliph | C-H aromatic | -CS-NH- |
|---|---|---|---|---|---|---|---|
| VIII | 3201.83-3167.12 | 1593 | 1635.64 | 1674.21 | 2854.65 | 3043.67-3008.95 | 3201.83-3167.12 |
| IX | 1245.2 | 1599.3 | 1616.8 | 1637.2 | 2939.3-2868.0 | 3072.2 | 3414.3-3236.8 |
| X | 1267 | 1599.0-1578.5 | 1651 | 1726.6 | 2939.3-2866.0 | 3066.2 | 3415.9-3224.3 |
| XI | 1244.3 | 1617.5-1594.9 | 1637.6 | 1663.6 | 2950.4-2871.4 | 3072.7 | 3477.0-3414.7 |
Table 3: IR spectral data (cm-1) of macrocyclic hydrazone (VIII, IX, X, XI).
1H-NMR Spectra of macrocyclic hydrazine (VIII, IX, X, XI)
Compound (VIII): The 1HNMR spectrum (Figure 5) of the Schiff base (VIII), showed that in the signals at 12.513 and 8.901 ppm were assigned to the protons of thioamide C-SNH and imine-CH=N groups respectively. Signals in the region 8.395-6.943 ppm were assigned to the aromatic protons. While the multiple signals at 2.508-3.361 ppm assigned to the protons (-O-CH2-) group [43].
Compound (IX): The 1H NMR spectrum (Figure 6) of the Schiff base (IX), showed that in the region 2.223-1.873 ppm were assigned to protons of methyl groups in two different environments [43]. The signals at 12.511 and 8.954 ppm were assigned to the protons of thioamide C-SNH and imine -CH=N groups respectively. Signals in the region 8.391-7.121 ppm were assigned to the aromatic protons. While the singlet signal at 4.147 ppm assigned to the protons (-O-CH2-) group.
Compound (X): The 1H NMR spectrum (Figure 7) of the Schiff base (X), showed that in the region 2.868-1.305 ppm were assigned to protons of methyl groups in two different environments [43]. The signals at 10.923 ppm were assigned to the protons of thioamide C-SNH. Signals in the region 7.664-6.978 ppm were assigned to the aromatic protons. While the singlet signals at 4.126-4.211 ppm assigned to the protons (-O-CH2-) group.
Compound(XI): The 1H NMR spectrum (Figure 8) of the Schiff base (XI), showed that in the signals at 13.133 and 9.035 ppm were assigned to the protons of thioamide C-SNH and imine -CH=N groups respectively. Signals in the region 7.923-6.079 ppm were assigned to the aromatic protons. While the singlet signal at 3.982-3.723 ppm assigned to the protons (-O-CH2-) group [43]. The other obtained values for 1-H-NMR chemical shifts of the compounds are given in the experimental section. The 1-HNMR spectral data of the new compounds showed in the Table 4. These data are in good agreement with those previously reported for similar compounds. These results strongly suggest that the proposed compounds have been formed [41-42].
| Schiff base | Chemical Shifts δ ppm | ||||
|---|---|---|---|---|---|
| (CH2-CH2-)n | -O-CH2- | C-Haromatic | CH=N | -CO-NH- | |
| VIII | ----------- | 2.508-3.361 (m,8H) | 8.395-6.943 (m,12 H) | 8.903 (2,2H) | 12.513 (s,2H) |
| IX | 2.223 - 1.873 (m,8H) | 4.147 (s,4H) | 8.391-7.121 (m,12 H) | 8.954 (s,2H) | 12.511 (s,2H) |
| X | 2.868 - 1.305 (s,8H) | 4.126-4.211 (s,4H) | 7.664-6.978 (m,12 H) | ---------- | 10.923 (s,2H) |
| XI | ---------- | 3.982-3.723 (s,4H) | 7.923-6.079 (m,16 H) | 9.035 (s,2H) | 13.133 (s,2H) |
Table 4: 1H-NMR Spectra of macrocyclic hydrazone (VIII, IX, X, XI).
Polarization measurements
Table 5 shows the corrosion Potential (Ecorr), corrosion current (icorr) and Tafel slopes (ba and bc) values of mild steel in 1M H2SO4. Solution in the absence and presence of inhibitor of all the three compounds at 303K calculated from (Figures 9-12). From Table 5 it is clear that compounds (VIII, IX, X, XI), offers maximum inhibition efficiency among the compounds Schiff bases derivatives and the studied compounds suppress the anodic reaction to greater extent than the cathodic one. This behavior is typical of mixed type inhibitors with anodic predominance. The difference in the efficiency is referred to the molecular structure effect, which have π-delocalized system of Schiff bases derivatives (C=S) and unshared pairs of electrons of N, O and S atoms that may cause the increasing or decreasing of the electron density on center of adsorption and leading to an easier electron transfer from the functional group (C=S group) to the metal, producing grater coordinate bonding and hence different adsorption and inhibition efficiency.
| Compound Name | -Ecorr/mv | icorr/ | ba /(mv.dec -1) | bc | Rp | IE(Using icorr) % |
|---|---|---|---|---|---|---|
| μA cm-1 | /(mv.dec -1) | |||||
| Blank | 480 | 480 | 6.38 | 12.43 | - | - |
| VIII | 520 | 60 | 8.18 | 11.82 | 349.83 | 87.5 |
| IX | 500 | 70 | 10.19 | 11.17 | 330.54 | 85.36 |
| X | 460 | 110 | 7.49 | 18.65 | 116.019 | 77.08 |
| XI | 418 | 175 | 4.55 | 25.78 | 95.95 | 63.54 |
Table 5: Corrosion kinetic parameters of mild steel exposed to 1 mol. L-1 H2SO4 solution in absence and presence of inhibitors.
Mechanism of corrosion inhibition by Schiff bases derivatives
The transition of metal/solution interface from a state of active dissolution to the passive state is attributed to the adsorption of the inhibitor molecules and the metal surface, forming a protective film. The rate of adsorption is usually rapid and hence, the reactive metal surface is shielded from the aggressive environment, Adsorption process can occur by electrostatic forces between ionic charges or dipoles of the adsorbed species (electrostatic attraction between the positively charged protonated nitrogen atom and negatively charged mild steel surface, cathodic sites) and the electric charge on the metal surface can be expressed by its potential with respect to the zero charge potential, Also the inhibitor molecules can be adsorbed species to the vacant electron orbital of low energy in the metal to form a coordinate type of link. Adsorption of inhibitor molecules is often a displacement reaction involving removal of adsorbed water molecules is often a displacement reaction involving removal of adsorbed water molecules from the metal surface.
Biological activity
During the last two or three decades, attention has been increasingly paid to the synthesis of macrocyclic hydrazone (VIII, IX, X, XI), which exhibits various biological activities including antibacterial, fungicidal, tuberculostatic and plant growth regulative properties [44]. It was judicious to investigate the synthesis of various new types of Schiff base and studied their antibacterial activity against four strains of bacteria (Bacillus subtilis, Escherichia coli, Staphylococcus aureus, and Salmonella typhi). The concentrations used for the screened compounds are 50, 100, and 200 μg/ml. Ciprofloxacin was used as reference standard while DMSO as control and inhibition zones is measured in mm. The new compounds were tested against one strain each of a gram positive and two gram negative. The test results shown in Table 4, a new compound was active against tested and another compound are no active. All compounds are not active when used in the concentration of 50, 100 μg/ml but active in the concentration of 200 μg/ml (Table 6; Figure 13).
| Shiff base | Bacteria | |||
|---|---|---|---|---|
| Gram negative | Gram positive | |||
| B. subtilis | S. aureus | E.coli | S. typhi | |
| VIII | 17 mm | 18 mm | 15 mm | 18 mm |
| IX | 18 mm | 16 mm | 16 mm | 19 mm |
| X | 18 mm | 15 mm | 12 mm | 17 mm |
| XI | ||||
| Control | 00 mm | 00 mm | 00 mm | 00 mm |
| Ciprofloxacin | 20 mm | 20 mm | 20 mm | 20 mm |
Table 6: Effect of new Macrocyclic hydrazone (VIII, IX, X, XI) on the growth of tested bacteria (conc. 200 μg/ml).
The compounds are new and were prepared for the first time. The new compounds were identified by melting point, elemental analyses 1HNMR, IR, LC-MS, spectral methods. The prepared compounds have been biologically screened i.e., studying their effects against two gram-positive, two gram-negative bacteria. The results show that their activities were found to vary from moderate to very strong. The results, it is obvious that all four studied compounds function as effective corrosion inhibitors in 1M H2SO2 medium with compounds(VIII, IX) being the best of four compounds.