Journal of Chemical Engineering & Process Technology
Open Access
ISSN: 2157-7048
ISSN: 2157-7048
Research Article - (2019)Volume 10, Issue 3
There is a cationic group that makes sure waterborne polyurethane self-emulsify on the main or side chain of cationic waterborne polyurethane (CWPU), which brings excellent function to cationic polyurethane, simultaneously it reduces water resistance. In this research, the good stability of CWPU was prepared as well as the water resistance and softness of cationic waterborne polyurethane film was improved. Elasticity, extensibility, weight gain rate, contact angle, TG, and structure of polyurethane had been tested and evaluated. Also, emulsion stabilities of polyurethane were tested, including storage stability, high-temperature stability, freeze-thaw stability, electrolyte stability, acid stability, and dilution stability; In addition to that, particle size and its distribution were tested. The test showed that the emulsion stability of polyester polyurethane illustrated a good result, while the mechanical property, water resistance, and water repellency were poor. Moreover, mechanical property and water resistance of polyether polyurethane film were good, but high-temperature stability, acid and alkali resistant, and water repellency of emulsion showed poor. For the synthesis of polyurethane, mixed soft segments of polyester (PE2348) and polyether (ZC330) were used, when mass ratio of (PE2348) to (ZC330) is not less than 1, that helped to improve the emulsion stabilities, increased contact angle, decreased particle size. But the water-resistance of film weakened when mass ratios of PE2348 to ZC330 were increased and the mechanical property was poorer. TG showed that thermal stability became poor. The introduction of hydrophilic chain extender (N120) can reduce the water resistance and water repellency of polyurethane. When the mass fraction of N-methyl diethanolamine (MDEA) was about 6% to 7%, the smallest particle size was 61 nm, and the water repellency was larger. The increase of MDEA content would reduce the water-resistance of the film. Finally the effect of hydrophilic chain extender on waterborne polyurethane was studied.
Cationic waterborne polyurethane; Preparation; Property; Organo-silicon; Mono-glyceride; Modification
Polyurethanes characteristic functional group is the repeated urehtane (-NHCOO-) on the main chain of the polymer. It is synthesized by the polymerization of isocyanate compounds and compounds with active hydrogen. Polyurethane has been widely and rapidly developed since its advent because of its excellent fatigue resistance, breathability, and physical and mechanical properties.
In 1937, Bayer, Germany, first prepared polyurethane resin and polyurea compound by the step-by-step addition reaction of isocyanate and polyol compound. In 1944, after the first pilot-scale successful synthesis of polyurethane resin, the polyurethane industry has been a new generation of development. However, the early preparation technology of polyurethane requires the use of a large number of organic solvents, including benzene, toluene, chlorobenzene, methanol and so on. The volatilization of these organic solvents not only pollutes the environment but also harms human health [1]. With the continuous improvement of people's quality of life and the stricter requirements of environmental regulations, the research on waterborne polyurethane became more and more in 1972 when Polyurethane Water Dispersions came out [2].
The polyurethane emulsion prepared by introducing hydrophilic ionic groups onto the molecular chain has stable properties, good film formation and low solid content of [3,4]. However, the size of the polyurethane emulsion obtained by adding emulsifier and protective colloid is larger. Because of the residue of emulsifier, the performance of polyurethane film is affected, the stability is poor, and the solid content is as high as 50% ~ 60% [4]. Generally, according to the different ionic groups in the main or side chains of polyurethane molecules, they can be divided into anionic polyurethane, cationic waterborne polyurethane, and non-ionic polyurethane. Anionic polyurethane is a chain extender containing carboxyl or sulfonate, cationic polyurethane is a chain extender containing tertiary ammonia or halogen group, and nonionic polyurethane is a chain extender containing polyethylene oxide.
Waterborne polyurethane with water as the solvent can maintain good performance, pollution-free, safe transportation, good working environment, safety, and low price. Therefore, waterborne polyurethane can be widely used in leather, furniture, construction, textile, automobile, printing, and even pharmaceutical industries. At present, there are many anionic waterborne polyurethane products in the market, but when cationic waterborne polyurethane is applied to leather, it can give the leather a soft, smooth feel and naturally rich appearance, which is incomparable with anionic waterborne polyurethane, because molecular chains contain hydrophilic chains and side chains. Ionic groups lead to the poor water resistance of polyurethane film, and the poor heat resistance caused by linear molecules of most waterborne polyurethane molecules hinders the development and application of cationic waterborne polyurethane.
Cationic waterborne polyurethane has good hydrophilicity because of its positive charge on the polymer chain. It is easy to disperse and stabilize in water. Waterborne polyurethane emulsion dispersing medium is water. During the drying process of polyurethane emulsion, the water molecules are not easy to evaporate and the drying speed is slow. Besides, cationic waterborne polyurethane has a linear structure and poor water resistance and solvent resistance. Therefore, most of the research focuses on improving the performance of waterborne polyurethane. At present, there are many studies on water resistance and storage stability of waterborne polyurethane at home and abroad. To obtain better water resistance and storage stability, the functional and structural modifications are generally carried out [5]. Structural modification is mainly carried out through the following aspects:
(1) Most of the cross-linked cationic waterborne polyurethanes have a linear structure, and their mechanical properties and breathability are poor. By introducing some trifunctional compounds through internal cross-linking to construct some network structures, the performance of these compounds can be improved; or using diplomatic cross-linking, the main diplomatic agent is water.
(2) Blending modification with different kinds of waterborne resins, such as acrylic emulsion, waterborne epoxy resin and so on, can complement each other according to their respective properties, and reduce their cost and improve their application performance.
(3) Modified polyurethane by copolymerization mainly focuses on the preparation of interpenetrating polymer network emulsion of polyurethane and acrylic acid. The copolymerization modified waterborne polyurethane has excellent properties of two kinds of resins and is generally used in dry paint, printing and other fields. Functional modification usually involves some functional compounds, such as epoxy resin modification, silicone modification, and acrylic acid composite modification.
Epoxy resin is a thermosetting plastic, which is widely used in various industries and has strong adhesion. Its price is low, the modulus is high, chemical stability is good, strength is high, insulation is good. These epoxy resins are being used in many fields such as machinery, electronics, coatings, and transportation, aerospace and so on. However, the application and development of epoxy resin are limited to a certain extent due to its brittle film formation and poor toughness. The properties of epoxy resin and waterborne polyurethane can be complemented by mixing and copolymerizing. The water resistance, chemical resistance, heat resistance and mechanical properties of waterborne polyurethane can be improved [6]. The structure of the epoxy resin contains -OH group, which can react with -NCO. The modification methods of waterborne polyurethane by epoxy resin include physical blending, graft copolymerization, and epoxy ring-opening. Modification of waterborne polyurethane by epoxy resin can obtain better results. However, the grafting amount of epoxy resin should not be too high, because the copolymerization of epoxy resin onto waterborne polyurethane will make the molecular weight increase rapidly and the viscosity increase, making emulsification difficult.
Zhou Haifeng and Wu Xiaoqing used epoxy resin to modify polyurethane, and the water-resistance of modified polyurethane was improved [7,8].
Organo-silicon modification is generally polysiloxane modification, because its molecule contains silicon elements, and has the advantages of inorganic and organic compounds. These compounds have good oxidation resistance and low surface energy. They have many excellent properties such as wear durability, biocompatibility, low-temperature flexibility and high-temperature stability [9]. Because of the existence of -OH group in polysiloxane series products; it can react with waterborne polyurethane by addition polymerization and chain extension. The water-resistance of modified polyurethane film has been improved [10-12]. In the thermodynamically unstable system of modified waterborne polyurethane synthesized, the very high degree of micro-phase separation will lead to poor mechanical properties, so it is necessary to adjust the hard and soft segments to obtain the appropriate degree of micro-phase separation of waterborne polyurethane.
Acrylate is widely used in automobile, metal tools, textiles, coatings, and other industries. It has many excellent properties, such as excellent weather resistance, good flexibility and light resistance, and excellent acid and alkali corrosion resistance. By modifying the waterborne polyurethane, the water resistance, heat resistance, tensile strength, wear-resistance and impact strength of the product have been improved [13,14].
The main methods of acrylate modification of waterborne polyurethane are as follows:
• The physical blending of PU and PA emulsion. Simple physical blending results in poor compatibility due to hydrogen bonding between compounds, or chemical crosslinking agent is introduced, but the properties are not satisfactory.
• Waterborne polyurethane emulsion containing core/shell structure can be prepared by copolymerization of active PU emulsion and acrylate emulsion.
The double modification of organo-silicon and acrylate is to combine waterborne polyurethane with organo-silicon and acrylate monomers and take the water dispersion of waterborne polyurethane as emulsifier and reactant in the reaction to carry out graft copolymerization to form core/shell and interpenetrating network structure. Zhang Xiaolei and Wu Wenxin used this method to modify polyurethane, and the water resistance and adhesion of the modified emulsion increased [15,16].
Nanomaterials have special properties such as optical effect, surface effect and quantum size effect. The waterborne polyurethane is modified by nanomaterial through chemical reaction to enhance the self-cleaning ability of the coating, enhance rigidity and strength, improve the filling effect, improve the interface bonding ability of the primer coating, and the emulsion has good stability, excellent aging resistance, chemical resistance, wear-resistance, and antistatic properties, etc. [17-19].
The preparation methods of nano modified polyurethane elastomers (PUE) are mainly sol-gel, intercalation, blending, in situ compounding and so on [20].
In addition to the above modification methods, biomass polyols such as castor oil, sucrose, methoxy soybean oil, and air-dried vegetable oil can also be used. Biomass polyols as raw materials are not only cheap but also come from natural renewable, environmentally friendly and healthy, reduce pollution and reduce environmental pressure. Chaozhou et al. used castor oil to modify cationic waterborne polyurethane. Tests showed that the introduction of castor oil could improve the water-resistance of the film. Qiu Wei and so on, through the glycerol alcoholysis of air drying vegetable oil, and then introduced into polyurethane pre-polymer, self-emulsifying vegetable oil modified waterborne polyurethane emulsion. The research shows that the waterborne polyurethane emulsion modified by air drying vegetable oil is stable, with strong flexibility, high hardness, high adhesion, and strong water resistance.
Cationic waterborne polyurethane has cations on its molecular chain. The hardness of water has little effect on it in the course of use. It can also be used under acidic conditions. It is widely used in leather, coatings, textiles, and papermaking and has a bright market prospect.
Cationic waterborne polyurethane has good bonding force to chrome tanning and fabric tanning in leather. It can be absorbed by the fabric without penetrating agent in the reaction process. Compared with anionic waterborne polyurethane, cationic finishing agent is more flexible and natural and can improve fiber strength, filling leather to make it soft.
Traditional solvent-based polyurethane coatings are gradually replaced by waterborne polyurethane coatings in recent years due to the volatilization of chemical solvents, which causes environmental pollution. Cationic waterborne polyurethane leather coatings are widely used in plastic coatings, industrial coatings, and paper coatings because of their beautiful film, low-temperature resistance, and good chemical resistance. Cationic waterborne polyurethane has good wettability to hydrophobic fabrics because of its cationic group on its molecular chain. It can be used for bonding furniture, automotive interior parts, foam, ABS and so on. At present, the properties of single structure polyurethane resin can be improved by introducing functional molecular chains such as silicon-containing polymer chains and acrylic resins into polyurethane chains.
Waterborne polyurethane polymer structure has the adjustability of soft and hard segments. Various properties of waterborne polyurethane can be prepared by changing the types and proportions of soft and hard segments. The novelty of this work is that the application of waterborne polyurethane in fabric finishing agent which contains cationic group in the main chain can improve the hand feel, wrinkle resistance, waterproof and moisture permeability of the fabric. Because of its positive charge, it has the good anti-static effect, can improve the anti-pilling phenomenon of fabric, and the fabric after polyurethane treatment, improve the adhesion between fibers, and enhance the washing resistance. Meanwhile, this strategy might provide a novel method in terms of synthesizing cationic waterborne polyurethane which is synthesized with soft and hard segments.
Waterborne polyurethane can be used as glass fiberizing agent in the production and application of glass fiber. The addition of polyurethane emulsion to a wetting agent can not only improve the surface defects of glass fiber but also improve the clustering and chopping properties of glass fiber. Waterborne polyurethane can also be used in footwear materials for filling and surface finishing.
Because the molecular structure of cationic waterborne polyurethane is mainly linear, and the molecular chain contains hydrophilic links and ionic groups on the side chain, which results in the poor heat resistance and water resistance. At the same time, to improve the handle of cationic waterborne polyurethane and improve the mechanical properties of polyurethane film, a series of modified cationic waterborne polyurethane emulsions were prepared, and their properties and characterization were evaluated.
A detailed list of materials and methods is discussed in the following Table 1.
Laboratory reagents
| Name of reagent | Abbreviation | Reagent specifications | Manufacturer |
|---|---|---|---|
| Polyether three alcohol | ZC330 | industrial products | Guangzhou Jinwang Chemical Co., Ltd. |
| Polyester glycol | PE2348 | industrial products | Guangzhou Jinwang Chemical Co., Ltd. |
| Polyether glycol | N120 | Chemical purity | Guangzhou Shantou Xilong Chemical Plant |
| 1,4-butanediol | BDO | Analytical purity | China Pharmaceutical Group Chemical Reagents Co., Ltd. |
| Isophorone diisocyanate | IPDI | Chemical purity | Shanghai Jingchun Reagent Co., Ltd. |
| N-methyl diethanolamine | N-MDEA | Chemical purity | Aladdin Reagent (Shanghai) Co., Ltd. |
| Gamma-aminopropyl triethoxysilane | KH550 | Chemical purity | Wuhan Warren Silicone Co., Ltd. |
| Gamma-aminopropyl methyl diethoxysilane | KH902 | Chemical purity | Wuhan Warren Silicone Co., Ltd. |
| Glacial acetic acid | HAC | Analytical purity | China Pharmaceutical Group Chemical Reagents Co., Ltd. |
| Methyl pentadiamine | -- | Analytical purity | China Pharmaceutical Group Chemical Reagents Co., Ltd. |
| Hydroxyalkyl polysiloxane | PPC | industrial products | Shanghai Jingchun Reagent Co., Ltd. |
| A. Glyceryl Monosterate | GMS | Chemical purity | Xilong Chemical Co., Ltd |
| Stannous octanate | DBTDL | Analytical purity | Aladdin Reagent (Shanghai) Co., Ltd. |
| Ethylenediamine | TEA | Analytical purity | China Pharmaceutical Group Chemical Reagents Co., Ltd. |
| Polycaprolactone | PCL | industrial products | Shanghai Jingchun Reagent Co., Ltd. |
| Polycarbonate | PCD | industrial products | Shanghai Jingchun Reagent Co., Ltd. |
| N-methyl pyrrolidone | NMP | Analytical purity | China Pharmaceutical Group Chemical Reagents Co., Ltd. |
Table 1: Experimental reagents and raw materials.
Emulsion performance test
Particle size and size distribution of emulsion: The solid content of waterborne polyurethane emulsion was diluted to 1%, and treated in an ultrasonic oscillator for 10 minutes. The ultrasonic vibration frequency was 80Hz. The particle size of emulsion was tested by laser particle size analyzer; the temperature was 20 ± 5°C.
Dilution stability: At room temperature, dilute waterborne polyurethane emulsion to 3% of solid content, 20 ml emulsion in the test tube, static 72 hours. If there is no stratification, then the stability is good; and if stratified, then measure the upper emulsion and the lower layer volume ratio to explain its dilution stability.
Acid-base stability: At room temperature, acid and alkali was added slowly to the emulsion containing solid content of about 25% in 10 ml, shaking to observe whether there was precipitation or gel. The pH value range of the emulsion state was measured by pH.
Electrolyte stability: At room temperature, a latex sample of 16 ml concentration of 40g/L was added to the calibration tube of 20 ml, and then added the CaCl2 solution with the mass fraction of 0.5% to 4 ml. Shake it well and put it on the test tube rack. Observations being done after 1 hour, 24 hours and 48 hours, the change of emulsion were observed, such as stratification, precipitation, flocculation and so on.
Freeze-thaw stability: 5 ml waterborne polyurethane emulsion is used to freeze in low-temperature refrigerator at -15°C for 16 hours and melt in a centrifuge tube at a constant temperature. Then the test sample was removed and defrosted at 20°C for 8 hours. This cycle was repeated 5 times, and it is assured to be qualified when there is no demulsification without gel formation.
High-temperature stability: The emulsion sample containing solid content of 25% was placed in the test tube, and 5D was placed in a constant temperature oven at 60°C. The change of the state was observed. If there was no change, it was qualified. If precipitation or gel was formed, the stability of 10G was poor.
Storage stability: At room temperature, the storage stability was simulated by centrifuge accelerated precipitation test. A certain amount of emulsion containing 25% solids was placed in a centrifuge test tube and centrifuged at 3000 rpm speed to centrifuge for 15 minutes. If there was no precipitation, it could be considered that there was a storage stability period of 6 months.
Testing of film properties
Water-resistance test of the film: The 10 g containing waterborne polyurethane emulsion with uniform solid content was poured on the 10 cm-10 cm flat bottom PTFE plate. The 5D was dried at room temperature and dried into the film. Then placed in the oven at 80°C, 24 hours was dried at 100°C, and 5 minutes was treated at 140°C. Finally, 1D was dried in the drying bottle of anhydrous copper sulfate.
Cut the film of 2 cm × 2 cm and call it m1. Immerse it in deionized water at 20+5°C for 24 hours. After quickly drying the water on the surface of the film with filter paper, call it m2. The weight gain rate of the film is as follows:
Testing of film contact angle: At room temperature, the static contact angles of waterborne polyurethane film at 0, 1, 2, 4, 6, 8 and 10 minutes were measured by contact angle tester.
Structural characterization analysis
FT-IR analysis: The polyurethane film was sheared and pressed with potassium bromide, and then tested by Nicolet iS5 infrared spectrometer at room temperature. The scanning range was 400-4000 cm-1.
Thermogravimetric analysis (TG): The water-borne polyurethane film was characterized by thermogravimetric analysis. The heating range was 50-600°C and the heating rate was 10°C/minute.
Cationic waterborne polyurethane
Two cationic water-borne polymers were synthesized by using 58% of polyester diol (PE2348) and ZC330 of polyether triol as soft segments, 30% of IPDI of isophorone diisocyanate as hard segments, 6% of BDO of 1,4-butanediol and N220 of polyether diol as chain extenders, and 6% of MDEA of N-methyl diethanolamine as cationic chain extenders. Polyurethane, the chemical structure of WPU is shown in Figure 1.
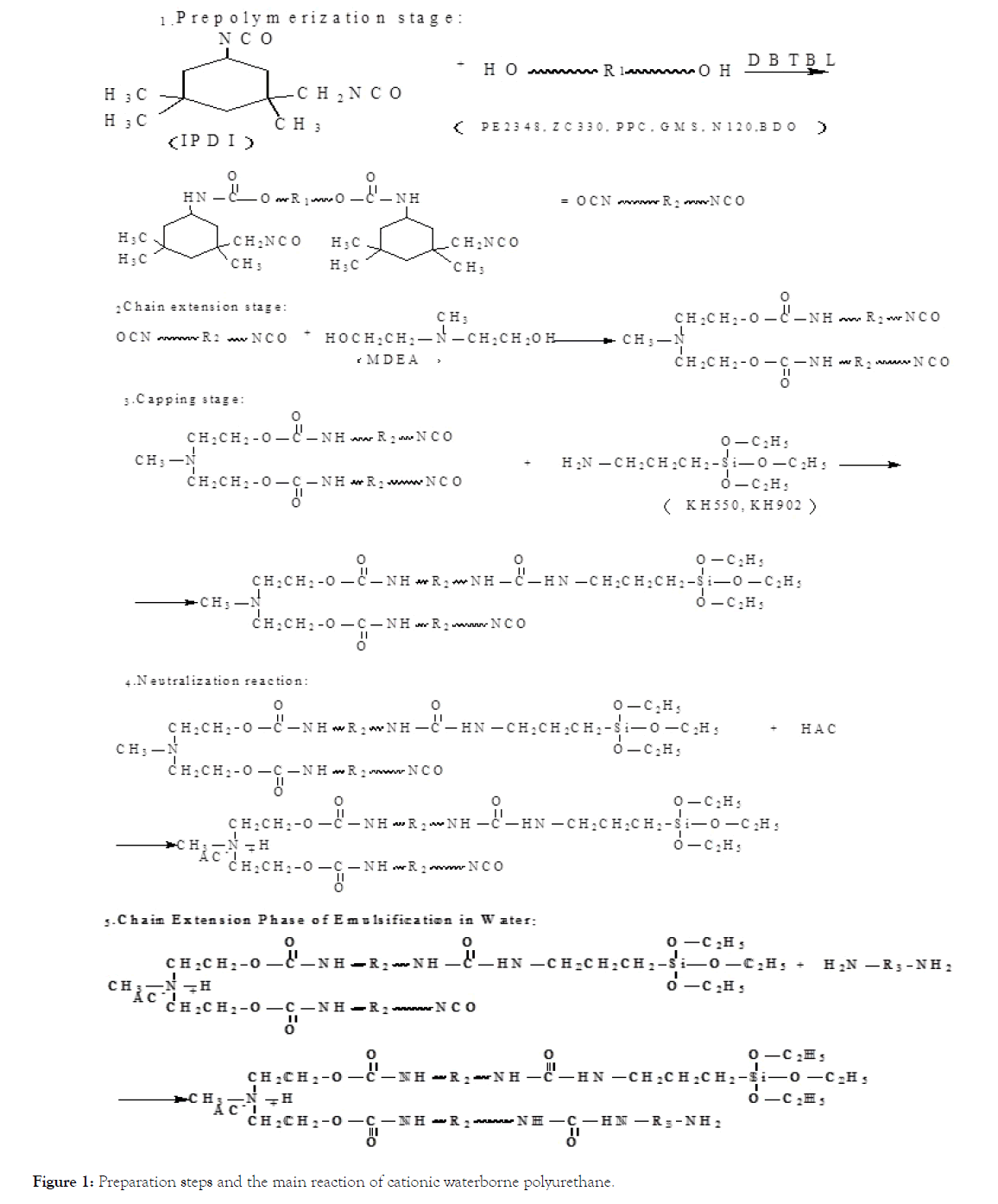
Figure 1: Preparation steps and the main reaction of cationic waterborne polyurethane.
Properties of emulsion and film: From the Table 2, the appearance of A-2 waterborne polyurethane emulsion is milky white, while the appearance of A-1 waterborne polyurethane emulsion is translucent. This is because although the hydrophilicity of A-1 is worse than that of A-2, A-1 is a linear structure and A-2 is a cross-linked structure. Therefore, the emulsion size of A-1 is smaller than that of A-2, and the smaller the emulsion particle size, the more transparent the emulsion appearance is.
| Groups | Emulsion appearance | Film color | Film feel | Film elasticity | Film extensibility |
|---|---|---|---|---|---|
| A-1 | Translucent | Transparent | Soft and sticky | Good | Different |
| A-2 | Milky white | Transparent | Slightly soft and non-sticky | Excellent | Excellent |
Table 2: Effect of different soft segment on properties of emulsion and film.
The film handle of A-2 is worse than that of A-1 because although polyether-based waterborne polyurethane contains a large number of C-O-C groups and the single bond is easy to rotate, ZC330 is a cross-linking structure of waterborne polyurethane synthesized as soft segment; although ester-based cohesion energy is much higher than that of ether group in polyether polyols, the structure of polyurethane synthesized is linear. The structure, molecular chain flexibility is better than A-2 and the polyurethane film is softer.
The elasticity and elongation of A-2 film are good because A-2 is cross-linked polyurethane. Because of the uniform distribution of cross-linking structure between soft and hard segments, the soft and hard segments move with each other and the force is distributed at each cross-linking point, then each cross-linking point plays a smaller role and can play a larger role when the tension is lost. The elasticity and elongation of A-2 film is good because the cross-linking structure of the molecular chain can be restored to its original state when stretched. The elasticity of A-1 film is good because of the high cohesion energy of ester group, the stronger hydrogen bond formed in the soft segment micro-region, and the hydrogen bond formed between ester group and carbamate group, which promotes the mixing between soft and hard segments and makes the strength of polyurethane higher.
So the elasticity of polyester polyurethane film is good, but its elongation is poor.
Emulsion stability (Table 3) in addition to the high-temperature stability and acid-base stability of A-2 waterborne polyurethane, other waterborne polyurethane emulsions are very stable. This is because the polarity of the ester group is large, the intermolecular force is large, and the thermal stability is good, so the high-temperature stability and other properties of A-1 polyurethane are good. Polyurethane A-2 is a cross-linking structure. In high-temperature environment, the polyurethane polymer chain moves, the molecular chain expands, the particle size becomes larger, and the molecules adhere to each other. Moreover, the hydrophilicity of the ether group is relatively poor, and the dispersion of polyurethane molecules is even worse under high temperature. Besides, the hydrolysate condensation of the capping agent makes A-2 gel at high temperature. When the pH value of the system is changed, the number of ionic groups on the polyurethane molecular chain will increase or decrease. Polyurethane A-2 is a body structure. The interaction force between the molecular chains will change due to the increase or decrease of the number of ionic groups, which makes the crosslinking system unstable, so the acid and alkali resistance of polyurethane A-2 is poor.
| Groups | Storage stability | High temperature stability | Freeze-thaw stability | Electrolyte stability | Acid and alkali resistance stability | Dilution stability |
|---|---|---|---|---|---|---|
| A-1 | No precipitation | No precipitation | No precipitation | No precipitation | pH 1-10 | No precipitation |
| A-2 | No precipitation | Gel | No precipitation | No precipitation | Acid and alkali resistance | No precipitation |
Table 3: Emulsion stabilities in addition to the high-temperature stability and acid-base stability.
Particle size and size distribution of emulsion: The particle size of waterborne polyurethane emulsion was tested by Nanotrac laser particle size analyzer, as shown in Figure 2.
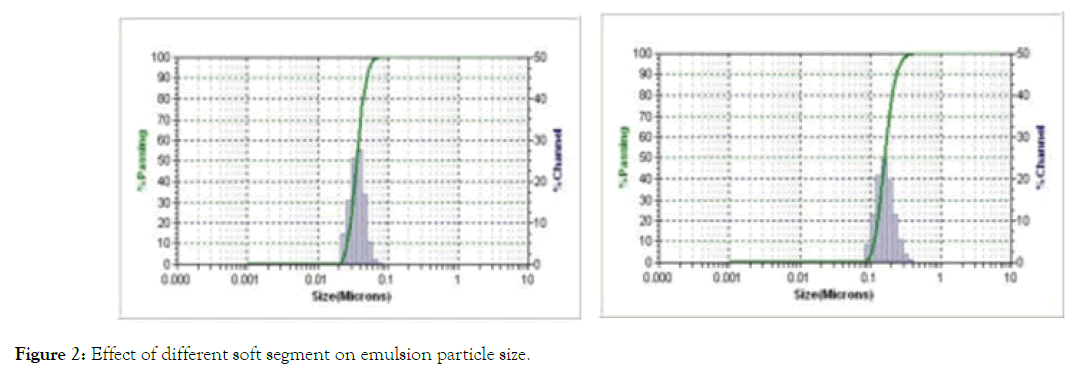
Figure 2: Effect of different soft segment on emulsion particle size.
From Figure 2, we can see that the average particle size of A-1 is 36.4 nm, which is distributed between 26.52 - 53.1 nm, and the distribution range is narrow. The average particle size of A-2 is 158.5 nm, which is between 113.2 - 262.7 nm. A-2 has a large particle size and uniform particle size distribution.
This is because the soft monomer of A-2 waterborne polyurethane is polyether triol, the molecular structure of polyurethane is cross-linked network structure, and the polyether structure of A-2 waterborne polyurethane is too hydrophilic, resulting in swelling, viscosity increase, and particle size coarsening. The polyester structure of A-1 waterborne polyurethane is moderately hydrophilic, and the linear structure of the A-1 polyurethane molecule. The hydration of polyurethane molecule with water makes the polyurethane micro-phase disperse, the number of latex particles increases and the particle size decreases.
Water-resistant stability of film: From Figure 3, it can be seen that the water-resistance of A-2 film is better, the 24 hours water resistance weight gain rate is 37%, the water-resistance of A-1 is poor, and the 24 hours water resistance weight gain rate is 90%.
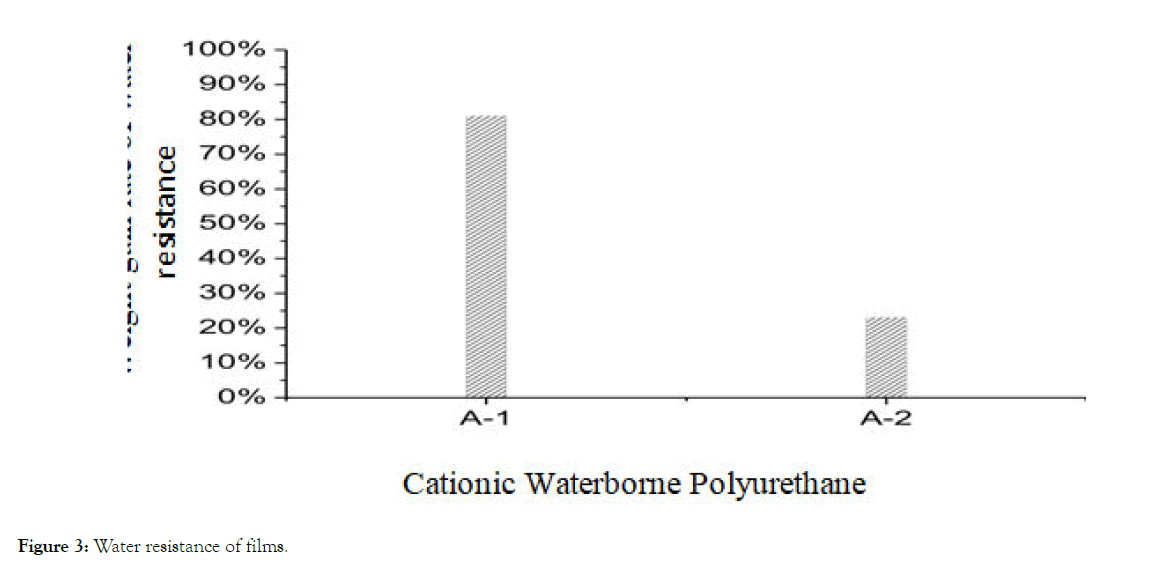
Figure 3: Water resistance of films.
The reason for the poor water resistance of linear waterborne polyurethane film is that hydrophilic micro-zones are formed due to the polarity of hydrophilic segments in the film-forming process of polyurethane water dispersions, and hydrophilic micro-zones are easy to swell under the action of water and reduce their mechanical properties. The ester group in linear polyester polyurethane is easy to hydrolyze, and the carboxylate ion on it further accelerates the hydrolysis of the ester group. At the same time, the micro-phase separation of linear polyester polyurethane is larger than that of cross-linked polyether polyurethane, the hard segments are relatively concentrated, the hydrogen bonds between hard segments are more, the density of hydrophilic groups is relatively large, the hydrophilicity is higher, the soft segments form a large number of amorphous regions, the hydrophilic groups provide the power for water molecules to enter the amorphous regions, and the water molecules are easy to enter. Chemical bond breaking occurs when the ester group reacts with the inner part.
Polyether polyurethane ether group is not easy to hydrolyze, has high crosslinking density, poor micro-phase separation, and water molecules are not easy to enter the polyurethane molecule. Therefore, the water-resistance of polyether polyurethane is better than that of polyester polyurethane.
Film contact angle: The static contact angle of waterborne polyurethane film was measured by contact angle tester within 10 minutes. The test results were drawn as follows:
As can be seen from the Figure 4, the film contact angle of polyurethane A-1 and A-2 is small, and between 50-85 degrees, the film contact angle of polyurethane A-1 is slightly larger than that of A-2, and the decreasing trend is large.
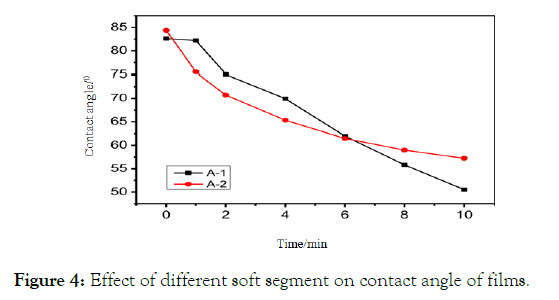
Figure 4: Effect of different soft segment on contact angle of films.
This is because the soft segment of polyester contains a large number of ester groups, and the hydrophobicity of ester groups is strong, but the polyester polyurethane molecule is linear structure, water molecules easily penetrate into the inner side of the film, resulting in a rapid decline in contact angle; while polyether polyurethane contains a large number of ether groups, the hydrophilicity of ether groups is strong, but ZC330 is a trifunctional compound. Water is not easy to penetrate the inner side of the film, so the decreasing trend of A-2 contact angle is similar to that of A-1.
Thermogravimetric analysis (TG): As can be seen from the Figure 5, the trends of the two thermogravimetric curves are almost the same. The mass loss begins at 220°C. This part should be the breaking of C-O bond in hard segment carbamate, which is decomposed into isocyanate and polyol.
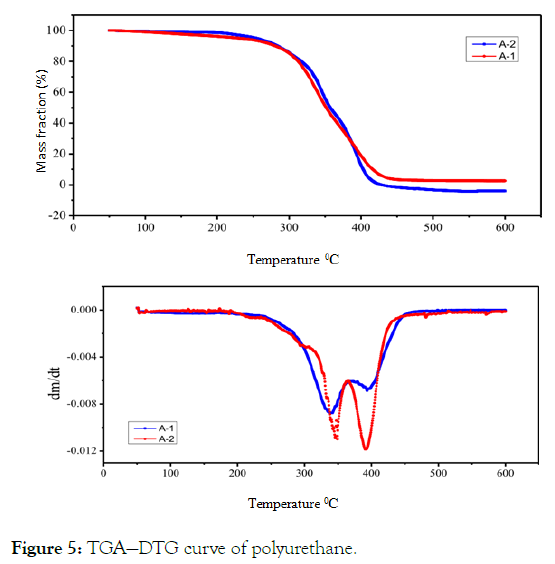
Figure 5: TGA—DTG curve of polyurethane.
Because A-1 is linear polyurethane and A-2 is bulk polyurethane, the thermal resistance of linear polyurethane is worse than that of bulk polyurethane. At first, the thermal decomposition of polyurethane molecular chains on the surface of A-2 film occurs, which is slower than that of A-1. When the molecular chains on the surface of A-2 film break down, the bulk structure is destroyed and the film is decomposed rapidly. Therefore, the thermal decomposition rate of A-2 film is slow first and then fast. In the second stage, the maximum weight loss temperature of A-1 is higher than that of A-2, and the maximum weight loss rate is lower than that of A-2. This is because the cohesion energy of ester group is larger, the ability of hydrogen bonding between molecules is stronger, and the polyester segment is easier to crystallize than the polyether segment, so the thermal decomposition temperature of A-1 in the second stage is higher and slower.
From the area analysis of the weight loss peak, the weight loss ratio of the soft and hard segments is the same as the total mass ratio of the soft and hard segments of the synthetic waterborne polyurethane.
FT-IR analysis: From Figure 6, it can be seen that the infrared spectra of A-1 and A-2 waterborne polyurethanes are almost identical, basically coincide, and there is no big difference. The absorption peak of A-1 at 1729.45 cm-1 is different from that of A-2, which is the C=O based stretching vibration absorption peak in polyester. There was no characteristic absorption peak of IPDI characteristic group -NCO near 2270 cm-1, and the reaction of -NCO group with -OH group occurred at 1563.16 cm-1. The strong absorption peak of NH-COO of carbamate bond indicates that IPDI has reacted with the hydroxyl group. The absorption peaks at 1699.34 cm-1 are C=O stretching vibration absorption peaks in polyurethane, C-O-C stretching vibration absorption peaks at 1129.12 cm-1 in polyester, polyether and urethane groups, and the absorption peaks at 3423.62 cm-1 in polyester polyols and PU is ester group frequency doubling absorption peaks. The merging peaks of NH stretching vibration absorption peaks, C-H stretching vibration and deformation vibration absorption peaks of CH2 and CH3 groups appeared at 2965.08 cm-1 and 1409.93 cm-1. The absorption peak near 1249.12 cm-1 is the symmetrical deformation peak of Si-CH3, and the expansion vibration absorption peak near 1033.23 cm-1 is the expansion vibration absorption peak of Si-O bond. In conclusion, IPDI has reacted with PE2348 and ZC330 monomers to produce polyurethane with siloxane termination.
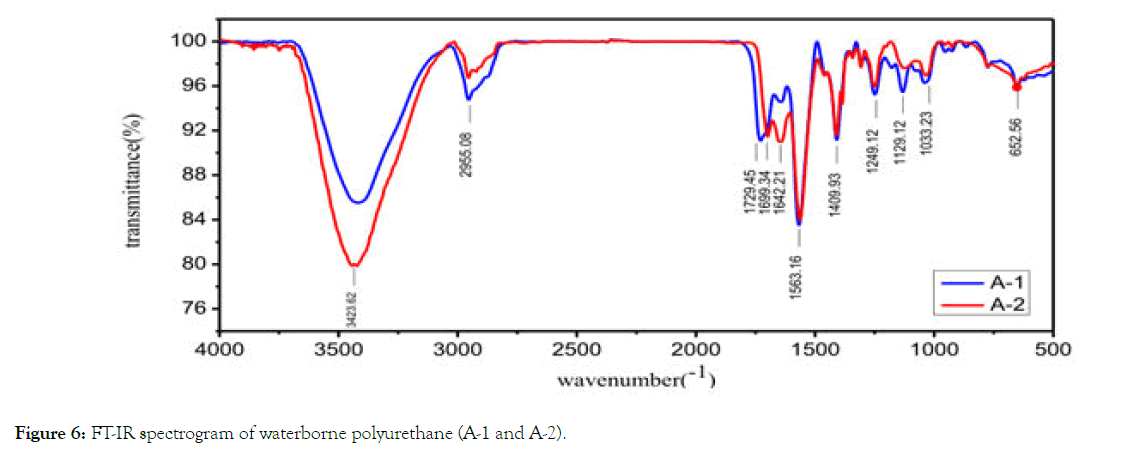
Figure 6: FT-IR spectrogram of waterborne polyurethane (A-1 and A-2).
Silicone Modified Polyurethane
It can be seen from Table 4 that after modification of polyester polyurethane and polyether polyurethane, hydroxyl alkyl polysiloxane PPC has a serious yellowing of the polyurethane film, a soft touch on the film, and no improvement in the elasticity and elongation of the film, while the appearance of the emulsion of polyether polyurethane gradually becomes transparent, and the color of the film gradually becomes yellow. The elasticity and elongation of the membranes are generally worse than those of pure ZC330 polyurethane.
| Groups | Emulsion appearance | Film color | Film feel | Film elasticity | Film extensibility |
|---|---|---|---|---|---|
| C-1 | Slightly transparent, Slightly yellow | Yellow | Soft | Different | Different |
| C-2 | Slightly transparent, Slightly yellow | Yellow | Soft | Different | Different |
| C-3 | Slightly transparent, Slightly yellow | Yellow | Soft | Different | Different |
| C-4 | Micro and yellow | Yellow | Soft | Different | Different |
| C-5 | Milky white | Transparent | Soft | Good | Similar |
| C-6 | Semi-permeable and turbid | Transparent | Soft | Good | Similar |
| C-7 | Yellow and turbid | Yellowish | Soft | Similar | Excellent |
| C-8 | Yellowish and semi-transparent | Yellowish | Soft | Different | Excellent |
Table 4: Effect of silicone modified polyurethane on properties of emulsion and film.
This is because the cohesion energy of ester group in polyester polyurethane is high, and hydrogen bond between the ester group and hard segment is weakened. Before using pure PE2348 as a soft segment in synthesis of cationic Waterborne Polyurethane, the compound showing poor elasticity and poor elongation, phase separation, good compatibility between hard and soft segments and high hardness. The hydroxyalkyl polysiloxane into the polyurethane soft segment chain, making the polyester soft segment ester-based density decreased as a whole, and organic silicon energy accumulation is small, micro-phase separation is serious, hardness decreased. Therefore, the elasticity and elongation of the film have not been improved. PPC contains a large number of Si-O-Si bonds, and the silicon-oxygen bonds have good flexibility, so the introduction of PPC makes the film more flexible.
The PPC was introduced into the cross-linked polyether polyurethane molecules, and the ZC330 content decreased, resulting in a decrease of crosslinking density. The introduction of PPC decreased the hydrophilicity of the molecules, and the swelling effect became smaller, so the appearance of emulsion changed and become gradually transparent. The introduction of hydroxyalkyl polysiloxane reduces the crosslinking density, resulting in the elasticity and extension of the film. The properties of polyurethane synthesized with ZC330 as a soft segment are much worse than that of pure ZC330. When the content of PPC continues to increase, the crosslinking of polyurethane will occur. The decrease of density and the low polarity of silicon soft segments result in the separation of hard and soft segments and the chemical interaction between hard and soft segments. When the force decreases, the elasticity of the film decreases and the elongation at break increases.
To sum up, the introduction of PPC made the polyester polyurethane felt soft, the appearance of the emulsion and the mechanical properties of the film had not been improved. The appearance and film of polyether polyurethane emulsion became yellow, the film felt soft, the elasticity of the film decreased and the elongation increased.
Emulsion stability
It can be seen from Table 5 that the hydroxyl hydrocarbon polysiloxane PPC is introduced into the polyester polyurethane soft segment chain, and its stability is good in all respects, and there is no precipitation and gel phenomenon. The polyether polyurethanes have high-temperature stability and gel, and all of the gels. The macromolecules of polyether polyurethane are relatively more compliant. They react slowly at high temperature and are not sensitive to heat. Therefore, the introduction of flexible PPC has little effect on its thermal stability. Its emulsion has poor heat resistance, but its acid and alkali stability is improved.
| Group | Storage stability | High temperature stability | Freeze-thaw stability | Electrolyte stability | Acid and alkali resistance stability |
|---|---|---|---|---|---|
| C-1 | No precipitation | No precipitation | No precipitation | No precipitation | pH2-12 |
| C-2 | No precipitation | No precipitation | No precipitation | No precipitation | pH2-12 |
| C-3 | No precipitation | No precipitation | No precipitation | No precipitation | pH2-12 |
| C-4 | No precipitation | No precipitation | No precipitation | No precipitation | pH2-12 |
| C-5 | No precipitation | Gel | No precipitation | No precipitation | pH2-12 |
| C-6 | No precipitation | Gel | No precipitation | No precipitation | pH2-9 |
| C-7 | No precipitation | Gel | No precipitation | No precipitation | pH2-14 |
| C-8 | No precipitation | Gel | No precipitation | No precipitation | pH2-12 |
Table 5: Effect of silicone modified polyurethane on emulsion stabilities.
Polyester polyurethane has a high ester group, high thermal stability, greater intermolecular force than polyether polyurethane has high bond energy, high thermal degradation temperature, and good thermal resistance. In this case, the hydrolysis and condensation of KH550 molecules are promoted, and the crosslinking density is further increased. The introduction of organo-silicon PPC improved the acid and alkali resistance of polyether polyurethane but did not improve the high-temperature stability.
Particle size and size distribution of emulsion
The particle size of waterborne polyurethane emulsion was measured by Nanotrac laser particle size analyzer, as shown in the following Figure 7.
Figure 7: Effect of silicone modified polyurethane on emulsion particle size.
According to Figure 7(a), with the increase of PPC content, the particle size of polyester polyurethane emulsion showed a decreasing trend. Overall, when the PPC content was 12%, the particle size of polyurethane emulsion was the smallest, and the size of emulsion particles was smaller after silicone modified polyester polyurethane.
This is probably because the siloxy bond in the PPC chain is longer, and the flexibility is better than that of the ester group. The cohesive energy is lower than that of the ester group, and the separation between the hard and hard segments is larger. When the PE2348 soft monomer and the cationic chain extension part can ensure enough hydrophilicity, the particle size of the emulsion decreases gradually.
From Figure 7(b), it is known that with the increase of PPC content, the particle size of polyether polyurethane emulsion increases first and then decreases. This is because when the content of PPC is low, the content of ZC330 is high at this time. The high crosslinking density of the membranes restrains the movement of hard and soft segments. The hard and soft segments are well miscible and the hydrophilic groups are not dense. The introduction of hydroxyalkyl polysiloxane reduces the polar groups of soft blocks and hydrophobic silicone chains. It reduces the hydrophilicity of polyurethane particles and increases the particle size of polyurethane emulsion. When PPC content continues with the increase of ZC330 content, the flexibility of the polyurethane molecular chain was improved and the crosslinking density decreased. The particle size of the emulsion decreased sharply. After that, the increase of PPC content and the decrease of ZC330 content resulted in a decrease of PPC content. Its hydrophobicity contributes most to the increase in particle size.
In summary, the introduction of PPC will reduce the particle size of polyester polyurethane and polyether polyurethane emulsion.
Water-resistant stability of film
Due to the high degree of micro-phase separation of Organosilicon Modified Polyester Waterborne polyurethane, especially when the content of organo-silicon is high, its water resistance is poor, and the film dissolves in water for several hours. Compared with the pure polyester soft segment before, the water-resistance of Silicone Modified Polyester waterborne polyurethane decreased significantly.
This may be due to the low polarity and low cohesion energy of hydroxyalkyl polysiloxane, which increases the micro-structure of waterborne polyurethane. Phase separation, hard and soft segment miscibility is not good. Although hydroxyalkyl polysiloxanes have strong hydrophobicity yet, they are comparatively better than hydroxyalkyl polysiloxanes. The hydrophilicity of the polyester soft segment and the hard segment is stronger. Water molecules enter the amorphous zone of polyester soft segment more quickly, and the water content in the amorphous zone of the polyester soft segment is higher than that in the amorphous zone of the polyester soft segment. The hydrogen bond between the molecule and the polar groups such as ester group and carbamate group weakens the hydrogen bond between the original polymer. The ester group is easy to hydrolyze, and the hydrolysate further promotes the ester group hydrolysis. So silicone modified polyester polyurethane film has poor water resistance.
As can be seen from Figure 8, compared with polyether polyurethane, with the increase of PPC content, the content of ZC330 decreases and the water-resistance of silicone modified polyether waterborne polyurethane film shows an improvement trend, and when the content of PPC is about 16%, the water-resistance of the film is the best.
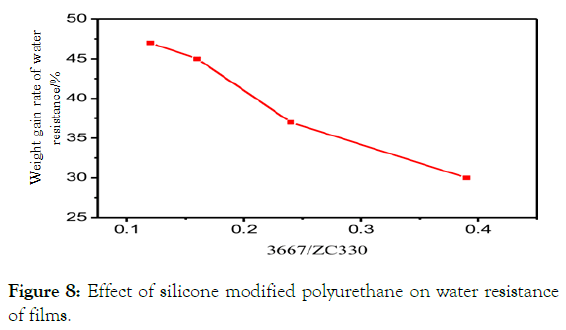
Figure 8: Effect of silicone modified polyurethane on water resistance of films.
When the content of organo-silicon increases, the cohesion energy of organo-silicon is low, the molecular chain is soft and the polarity is poor. It is easy to cause the formation of soft and hard segments, the separation of soft and hard segments, the pulling effect of hard segments on soft segments becomes smaller, the long silica chains of organo-silicon compounds are easy to migrate to the surface of the film, and the surface energy of organo-silicon compounds is very low and relatively low.
Strong hydrophobicity of the film enhances the water-resistance of the film.
Film contact angle
As can be seen from the Figure 9(a), the contact angle of silicone modified polyester polyurethane film has little change. The initial contact angle is between 80 and 95 degrees, which is larger than that of pure polyurethane film synthesized by PE2348. With the increase of organo-silicon compounds, the film contact angle decreases gradually, and the decreasing trend increases. This is because hydroxyalkyl polysiloxane has low surface tension and high surface activity. On the one hand, with the increase of hydroxyalkyl polysiloxane, the number of soft blocks of hydroxyalkyl polysiloxane in polyurethane molecular chain increases. On the other hand, the density of polar groups in the molecule decreases, and the cohesion energy decreases, which results in the surface of polyurethane film. As the surface tension decreases and the surface energy decreases, the contact angle of the film is larger. According to the analysis of water-resistance of Silicone Modified Polyester Waterborne polyurethane, the water-resistance of silicone modified polyester waterborne polyurethane is very poor. When measuring the contact angle of the film, water easily penetrates the film, resulting in a faster reduction of the contact angle.
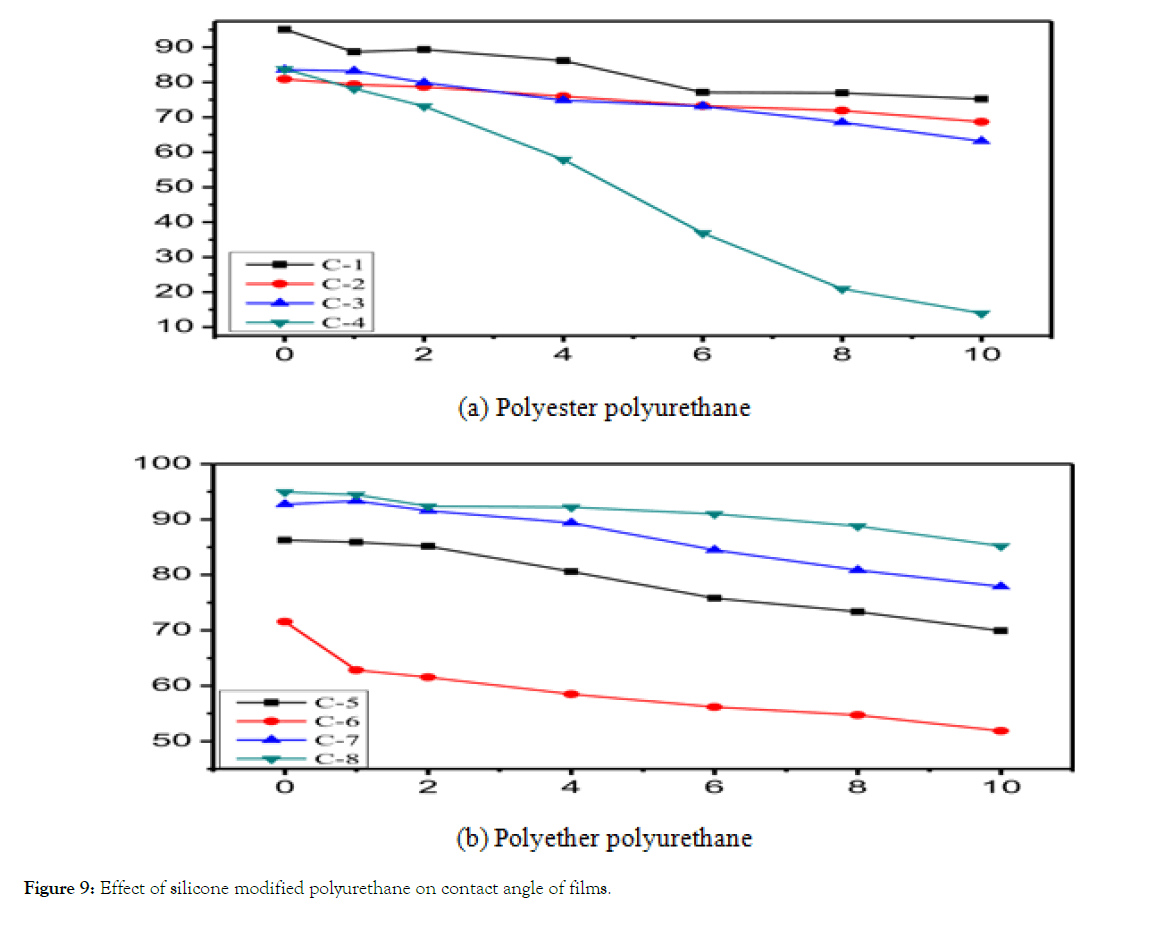
Figure 9: Effect of silicone modified polyurethane on contact angle of films.
As can be seen from Figure 9(b), with the increase of PPC content, the contact angle of organo-silicon modified polyether waterborne polyurethane shows a trend of decreasing first and then increasing. This is because, when the silicon content is low, on the one hand, the introduction of silicone chains leads to the confinement of the long silica chains in the film and the inability to migrate freely to the surface of the film; on the other hand, the introduction of silicone compounds leads to the separation of hard and soft segments, the relative concentration of polar groups and the crosslinking density. Reducing the contact angle of the film reduces the hindrance of water molecules entering the film. Reduce firstly and then, when the content of organo-silicon is high, on one hand, the crosslinking density of polyurethane decreases, the miscibility of hard and soft segments is poor, on the other hand, the polarity of organosilicon compounds is poor, which results in the separation of hard and soft segments, the pulling force of hard segments on soft segments decreases, and the long silica chains of organosilicon compounds migrate to the surface of the film for enrichment of organosilicon.
Because of the strong hydrophobicity and low surface energy of the compound, the surface energy of the film decreases, so the contact angle of the film increases subsequently, showing stronger water repellency.
Thermogravimetric Analysis (TG)
From Figure 10, it can be seen that the loss of a mass fraction of polyether polyurethane modified by organo-silicon is about 2% before 200°C, which is the mass loss of water in the film through evaporation. The mass loss near 220°C may be that ZC330 begins to decompose. The maximum decomposition rate occurring at 330 degree C-O bond cleavage in polyurethane hard segment carbamate group, decomposition into isocyanate and polyol, further decomposition into amines, olefins and CO2. The thermal decomposition of PPC at 400°C may be the thermal decomposition of PPC.
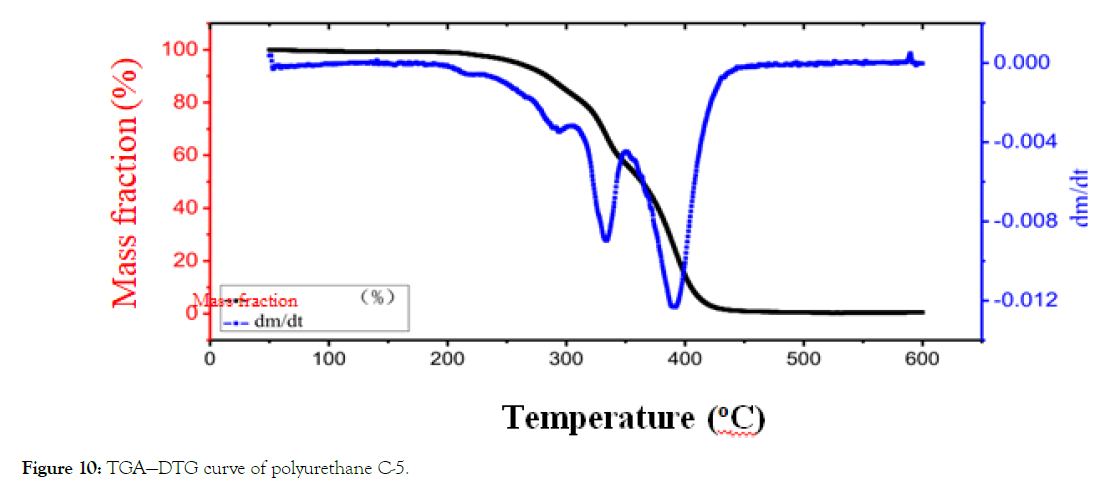
Figure 10: TGA—DTG curve of polyurethane C-5.
From the analysis of the shadow area of mass loss in the figure above, it can be seen that the proportion of the soft and hard segments and the thermogravimetric loss of organo-silicon compound accords with the proportion of the mass fraction of polyurethane synthesis in this chapter. The peak ratio of thermogravimetric DTG is narrower in Figure 10, which indicates that the micro-phase separation of hard and soft segments is serious, which is consistent with the viewpoint that the introduction of organo-silicon compounds into polyurethane chain leads to the increase of micro-phase separation of hard and soft segments.
According to results obtained, we can report that good tability, transparent appearance, sticky feel, good elasticity, and poor extensibility as well as a small phase separetion between soft and hard segments were resulted on the emulsion test of Polyester polyurethane. Due to a linear structure, water resistance and resistance of the film showed very poor index. Although mechanical properties, water resistance and compatibility of soft and hard segments, the water repellency is poor. With the increase of PE2348 content and the decrease of Polyether triol (ZC330) content, the stability of emulsion improved, the water repellency improved, but the mechanical properties and water resistance of the film became worse. When m(PE2348): m(ZC330)=1:1, the performance of mixed double soft segment polyurethane is the best. After modification of polyester polyurethane with hydroxyalkyl polysiloxane (PPC), the stability of the emulsion is soft and the water repellency is better. However, the emulsion and film become yellowing, the mechanical properties become worse and the water-resistance becomes worse. After polyether polyurethane modified by hydroxyl alkyl polysiloxane (PPC), the emulsion has good acid and alkali stability, soft handle, good water resistance, and water repellency, but the emulsion and film become yellowing, and the mechanical properties become worse. After PS modified polyether polyurethane, the adhesive film became sticky, the stability of acid and alkali was improved, the water resistance was general, but the emulsion became turbid, the mechanical properties became worse and the water repellency became worse. After the content of 3% GMS modified polyester polyurethane, the handle became smooth, the water resistance and water repellency of the film improved, but the appearance of the emulsion was improved. The mechanical properties of the emulsion become worse, and the emulsion stability becomes worse. After GMS modified polyether polyurethane, the emulsion stability was improved, the water-resistance of the film increased, but the appearance and mechanical properties of the emulsion became worse, and the water repellency was not improved. The water-resistance and water repellency of polyurethane film will be reduced by the participation of N120 in chain extension. It is suggested to remove the hydrophilic chain extender of N120. When the MDEA content is 7%, the particle size of the polyester polyurethane emulsion is 61 nm, the contact angle of the film is the largest, and the 10 minutes is reduced from 94.78 degrees to 82.83 degrees. The increase of MDEA content will lead to a decrease of water-resistance of the film. When the MDEA content is 6%, the water resistance and weight gain of the film is 39.12%. To sum up, the cationic chain extender content is moderate, and 6%-7% is the best.
Citation: Ismoilov K, Akram W, Chauhan S, Ergasheva K, Artikboeva R, et al. (2019) Synthesis and Evaluation of Properties of a Novel Cationic Waterborne Polyurethane Finishing Agent. J Chem Eng Process Technol 10:398. doi: 10.35248/2157-7048.19.10.398
Received: 29-Oct-2019 Accepted: 14-Nov-2019 Published: 24-Nov-2019 , DOI: 10.35248/2157-7048.19.10.398
Copyright: © 2019 Ismoilov K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.