Organic Chemistry: Current Research
Open Access
ISSN: 2161-0401
ISSN: 2161-0401
Research Article - (2015) Volume 4, Issue 1
Based on a rational approach using merely bioisosterism as euristic tool, we designed and tested a short series of congeners of 6-benzoyl-2(3H)-benzoxazolone as phenytoinergic lead. Among them, 5-benzoyloxindole showed an impressive activity in the Maximal Electroshock seizure test in mice at the same level of activity as phenytoin, carbamazepine and primidone, all these drugs nowadays considered worldwide as reference molecules, and only surpassed by ameltolide. Additional preliminary pharmacomodulations of this lead were unsuccessful. In view of its molecular concision and good druggability characteristics, 5-benzoyloxindole represents a valid platform for further medicinal chemistry elaborations.
Keywords: Antiepileptic activity; 5-benzoyloxindole; Phenytoinergic drugs; Bioisosteric substitution
Oxindole is a bicyclic heterocycle consisting of a sixmembered benzene ring fused to a five-membered pyrrolone ring. Oxindole derivatives are credited to possess a rather wide range of pharmacological effects which encompass antiviral, antifungal, antibacterial, antiproliferative, anticancer, anti-inflammatory, antihypertensive, antioxidant properties and interestingly enough anticonvulsant activities. In view of their interesting biological properties, the chemistry of oxindoles remains very important both for the synthetic organic and medicinal chemistry community [1,2] (Figure 1).
Oxindole can be regarded as a bioisosteric form of 2(3H)- benzoxazolone (1) which is fair to consider as a privileged template in drug design and discovery. Indeed carbamate oxygen in 3-position in 1 has been replaced by a methylene in 3. Among the various applications of this scaffold, two close derivatives of this template specially drew our attention because of their unprecedented pharmacological activities [3,4]. As a matter of fact, 6-benzoyl-2(3H)-benzoxazolone (1, 10194 CERM) and its sulfur bioisoster (2, S-14080) underwent extensive clinical trials until phase II as analgesic. S-14080 was found to inhibit not only the arachidonic acid inflammatory cascade but also to induce the release of an opioid peptide in periphery (however so far unidentified, possibly endomorphin, or nociceptin) [5]. A more recent work was published, expanding the structure-activity relationship of S-14080 as an analgesic compound [5]. 6-Benzoyl-benzoxazolin-2- one (1) and 6-benzoyl-benzothiazolin-2-one (2) also served as lead structures in the design of antiviral compounds, particularly targeted against HIV and CMV species [6].
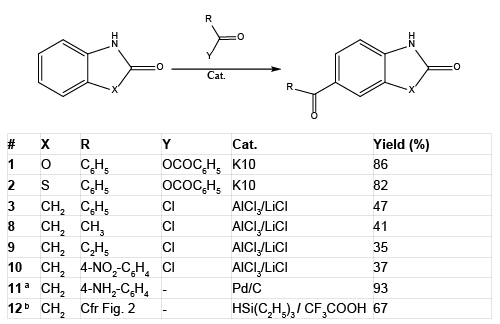
In an effort to further extend the medicinal chemistry of this useful scaffold and aiming at obtaining a useful antiepileptic drug with a phenytoinergic profile, we endeavored at synthesizing 6-benzoyl- 2-oxindole and testing its potential as an antiepileptic drug in a set of classical pharmacological assays (i. e. the Maximal Electroshoch Seizure test (MES), the subcutaneous pentylenetetrazole threshold test (scMet), and the Rotorod test (Tox). The term phenytoinergic refers to a compound with an anticonvulsant profile which is selectively active in the MES but totally devoid of activity in the scMET test. The straighforward rationale behind our approach was based on the use of bioisosterism, which has proved to be an efficient tool in drug design. Compounds 1, 2, and 3 were indeed obtained by the very same approach as explained above (Table 1).
 |
| aobtained by catalytic hydrogenation of 10 bobtained by transfer reduction of 3 |
Table 1: Synthesis of acylindolinones and related compounds
Most canonical phenytoinergic drugs contain on one hand a ureide moiety (i.e. phenytoin and carbamazepine). However, our oxindole derivative does not, and this remained for us a long standing question mark. It has been known on the other hand that biosiosteric replacement of one nitrogen of the ureido moiety can be effected using a methylene, an oxygen or a sulfur as exemplified by antiepileptic cyclic succinimides, 2.4-oxazolidinediones or 2-thiazolidine-2,4-diones or more recently by linear N-benzyloxycarbonylglycinamides [7,8]. There was therefore much precedence in the literature regarding our design approach herein explored. However, we would like to stress out the molecular concision of our title compound (MW=237.25), its low intrinsic lipophilicity (logP = 1.96), and the paucity of heteroatom’s (= 3) and rotable bonds (=2). Considering Lipinski’s and Weber’s rules, compound 3 thus has got all the noble attributes to become an attractive lead compound in the challenging task of designing new antiepileptic drugs with dual mode of action. In this connection, let us keep in mind that 2(3H)-benzoxalones and 2(3H)-benzothiazolones were successfully employed in the design of ?-1 anticonvulsant compounds [9,10]. As our lead (3) derives from these templates by bioisosteric substitution, we could infere that 3 can be launched successfully in such an enterprise.
Another aspect motivating this project was based on the fact that most significant lead compounds in the field of drugs acting on the sodium voltage dependant channels show to some extent major toxicity: for example, both phenytoin and carbamazepine are indeed strong cytochrome P450 inducers. This may be ascribed to their diphenylmethane-like structure which allows for extensive C-hydroxylation via an arene-oxide-mediated metabolisation pathway [9,11]. To our point of view, modification of these leads aiming at diminishing electron-density around the aromatic rings was considered as an important structural feature. Compounds such as ameltolide (4) [10-12] or their phthalimide analogues are flanked by an amino electrodonating group and therefore do not meet our structural stringent requirement. As a proof of concept, let us consider that ameltolide was stopped in preclinical developments due to toxicity (lung fibrosis) in animal species. It should be noted also that already at the time of Merrit and Putnam who conceived phenytoin; 4-aminobenzophenone was among the few twenty compounds which were selected for initial screening in their model of kindling in cat [13]. Pursuing along this line, we used this canonical concise template diminishing however electron density by including the 4-nitrogen position in an amide containing heterocycle, i.e. an oxindole heterocyclic system or its bioisosteric equivalents (Figure 2).
This design accomplished, the key problem was to access this target structure. Close examination of the literature revealed very few entries into this structure. Most trustful references describe the 7-benzoyloxindole, sometimes erroneously termed as a 3-benzoyloxindole derivative [14,15]. The target compound was incidentally synthesized in 1976 and its synthesis was reproduced in 2003 using a somewhat improved method. Various efforts were made to further improve access to this compound using a straightforward Friedel-Crafts benzoylation process of oxindole along with some known classical Friedel-Crafts catalysts [16,17].
To our pleasure, we were able easily to reproduce the 2003 Friedel- Crafts procedure but in our hands none of our other attempts were totally or partially satisfactory [18]. For example to our dismay, the reaction does not work unfortunately using the couple C6H5COOH: PPA (polyphosphoric acid) or the less convenient benzoic anhydride: hydrofluoric acid. The only improvement, we could bring about, was to add LiCl to the reaction mixture using DMF, AlCl3 complex as catalyst (Cfr experimental section). Indeed, lithium cation is an oxophilic reagent and therefore is endowed to efficiently catalyze the Friedel- Crafts reaction by polarizing the carbonyl group of the acyl chloride [19]. In this paper, we report the synthesis of 1 and 2 which were needed for biological testing using an improved method. Compounds 4, 5, 6, 7, 13, 14 and 15 were available from previous publications. Newly synthesized compounds (1-3, 8-12) are reported in the synoptic Table 2.
| # | MES ED50a | scMET ED50a | TOX TD50a |
|---|---|---|---|
| 1 | > 250 | > 250 | - |
| 2 | > 250 | > 250 | - |
| 3 | 11.2 | > 250 | 32 |
| 4 | 3.5 | - | 40 |
| 5 | 10.0 | > 250 | 80 |
| 6 | 38 | > 250 | - |
| 7 | 32 | > 250 | - |
| 8 | > 250 | > 250 | - |
| 9 | > 250 | > 250 | - |
| 10 | > 250 | > 250 | - |
| 11 | > 250 | > 250 | - |
| 13(Phenobarbital) | 9.1 | 11.6 | 61 |
| 14(Carbamazepine) | 8.5 | > 250 | 813 |
| 15(Primidone) | 6.2 | 15.4 | 233 |
Table 2: Antiepileptic Potencies of Phenytoin-like Compounds
As is evidenced in Table 2, among the compounds evaluated in our comparative tests the target compound 3 was found exceptionally active in the MES test and in the range of ED50’s very similar to leading drugs such as phenytoin and carbamazepine and by far much more active than phenytoin single amide bearing pharmacological probes 5 and 6 or the succinimide 7.
Interestingly enough, 3 was inactive in the scMET test and qualifies therefore as a phenytoinergic compound as enounced above. Encouraged by the good level of activity of 3 in the MES test, we synthesized two simple analogs, i.e the 5-acetyl and 5-propionyloxindole (8 & 9), which proved inactive both in MES and scMET tests. Another attempt to improve lead 3 was based on the possibility that 3 and ameltolide (4) could potentially belong to the same pharmacophoric family. Accordingly, 5-(4-aminobenzoyl) oxindole (11) was synthesized but did not exhibit significant activity both in the MES and scMET tests. Apparently, 5-benzoyloxindole (3) pharmacophore does not respond to the same structure-activity relationship as that of ameltolide (4). Finally, the ketonic carbonyl of 3 was reduced to give 5-benzyloxindole (12) which also proved equally inactive.
The present work reinforces the idea that the pharmaphoric requirements in phenytoinergic compounds are very stringent. Let us keep in mind that 6000 variants of phenytoin have been synthesized so far and none was show superior to the original lead.
Considering the general structure-activity of phenytoinergic antiepileptic drugs and based on a rationale using 6-benzoyl-2- (3H)-benzoxazolone (1) template as lead, we designed and tested a short series of bioisosteric modifications of this lead. Among them, 5-benzoyloxindole (3) was the only compound which showed an attractive phenytoinergic profile with an impressive ED50 value in the MES test of 11.2 mg/kg. In view of its molecular concision and good druggability characteristics, we truly believe that for 3 constitutes a pivotal platform for further creative pharmacomodulations.
General Procedures
Melting points (uncorrected were determined in open capillary tubes using a Büchi SMP 20 melting point apparatus. IR spectra were recorded using a dispersion of the product in KBr disks by means of a Perkin-Elmer Model 297 spectrometer. 1H and 13C NMR spectra were recorded using an AC 300P Brüker spectrometer. The NMR spectra were recorded at ambient temperature using tetramethylsilane (TMS) as internal reference. All compounds reported had IR, 1H and 13C NMR, MS, and elemental analysis data consistent with their structure. The experimental elemental analysis figures were found within 0.4% of the calculated values. Thin layer chromatography analyses were performed on Merck TLC plates (silica gel, 60F 254, E. Merck, Darmstadt, ref. 5735). All compounds reported here were found chromatographically homogenous in two standard solvents, i.e. acetone/toluene/cyclohexane (5:2:3, v/v/v) and methanol/chloroform equilibrated with ammonia (1:9, v/v). High-performance liquid chromatography conditions were: C-18, methanol: water (75:25, v/v), 1, 5 ml/mn. All compounds reported were found homogenous under these HPLC conditions. All reagents were purchased from Sigma/Aldrich. Other reference compounds were available from previous studies [9,17].
Synthesis of Reference Compound (1)
A solution of 1.35g of 2(3H)–benzoxazolone (1, 10mmol) and 2,49 g of benzoic anhydride (11 mmol) in 100 cm3 of anhydrous CH2Cl2 was treated with 1g of K10 Montmorrillonite and refluxed for 4h. After cooling, the mixture was diluted with 150 cm3 CH2Cl2, filtered over a bed of celite, washed with 5% Na2CO3solution, dried over MgSO4, and evaporated in vacuo to give a residue which was recristallized from ethanol to give 1.98 g (83%) of analytically pure 2 ( TLC, HPLC, mp, 170-172°C, 172-173°C). This material had IR, 1H, and 13C NMR spectroscopic data identical to those previously reported by Ucar and Cotelle [10]. In the same way was obtained compound 2 (yield = 82%).
5-Benzoyl-1, 3-dihydro-2H-indol-2-one (3)
To a mixture of 53.3 g of AlCl3 (0.4 mol), 1.68g (0.4 mol) of lithium chloride and 8.6 cm3 of DMF (115 mmol), heated and stirred mechanically at 50°C (oil bath), 5.34 g of 2-indolinone (14 mmol) were added in one portion. After a homogenous paste was obtained, 7.0 cm3 of benzoyl chloride (60 mmol) were added dropwise over 30 mn. When the addition was completed, the dark red mixture was stirred for another 3.5 h at 85°C and poured, while hot, onto 1 kg of ice containing 50 cm3 of concentrated HCl. The resulting precipitate was stirred for 2 h, filtered, washed copiously with distilled H2O, dried, and recrystallized from methanol to give 4.48g (47%) of 3 (mp 205- 206°C, 204-205°C) [20]. IR (KBr): ν = 3308(NH), 3062 (arom. CH.), 2916 (aliph. CH), 1705 (ketone C=O), 1641 (heterocycle C=O) cm-1; 1H NMR (CDCl3) δ=9.10 (s, NH), 7.70 m, H4, H7, H1, H5’), 7.50 (m, H3’), 6.90 (dd, J6-7 = 8.0Hz, J6-4 = 0.9 Hz, H6), 3.55 (s, CH2) ppm.13CNMR (CDCl3) δ 35.42, 126.00, 126.11, 128.41, 129.12, 130.11, 131.17, 132.02, 137.76, 148.09, 177.03, 195.01ppm. Compounds 7 and 8 were synthesized in the same way. 7(mp 223-224°C) 1H-NMR δ 10.76, (s, NH), 6.89-7.84 (m, 3H), 3.55(s, 3H) 2.49 (s, 2H). 13C-NMR δ 26.28, 35.42, 108.59, 124.27, 125.95, 129.23, 130.53, 148.24, 176.10, 196.15, ppm 8 1 H-NMR δ 10.7 (s, NH), 6.90-7.81 (m, 3H), 3.55(s, 1H), 2.95 (qd, 2H), 1.07 (t, 3H). 13C-NMR δ 8.34, 30.70, 35.45, 108.69, 124.01, 125.97, 128.81, 130.22, 148.03, 176.28, 199.02.
5-(4-aminobenzoyl)oxindole (11)
The 4-nitroprecursor was synthesized in 37% yield using essentially the same method as for 3 (mp 247-248°C from ethanol, 1H-NMR δ 10.90 (s, NH), 6.99-8.38 (m, 7H), 3.58 (s, 2H); 13C-NMR 35.42, 108.84, 123.49,125.98,126.37,129.02,130.09,131.58,143.68,148.95,149.01, 176.62,193.25 ppm. This material (1.0g, 4 mmol) was dissolved into 50 mL of 2-propanol and 10 mL of cyclohexene. To the resulting solution was added 0,4g of Pd/C. The suspension was refluxed for 6h, filtered and concentrated to one third to give crystals of the title compound (mp 210-211°C). 1H-NMR δ 10,72(s, NH), 6,68-7,54(m, 7H), 3,89 (br, 2H), 3,55 (s, 2H); 13C-NMR δ 35,52, 108, 30,113,35, 125,45, 125,68, 127,67, 129,98, 131,63, 132,01, 141,69, 146,90, 176,53, 192,67. This material was recrystallized from ethanol to give the analytically pure compound.
5-Benzyloxindole (12)
To a solution of 5-benzoyloxindole (3.0 g, 12 mmol) in 7.5 ml of dry trifluoroacetic acid was added dropwise under stirring at room temperature triethylsilane (3.5 g, 22 mmol). After 2h, another 3.5 ml of triethylsilane was added in one portion and the reaction mixture was left standing for 30h, after which time it was treated with 250 g of ice water. Mechanical stirring for 30 mn gave a thin white precipitate which was filtered on a Büchner funnel, dried in vacuo, and recrystallized from ethanol to give the title compound.Yield: 67% (mp 157-158°C, 1H-NMR δ 6.73-7.22 (m, 8H), 3.85(s,2H), 3.41(s,2H); 13C-NMR δ35.68, 40.72, 108,88, 124.72, 125.77, 125.93, 127.50, 128.29, 128.50, 134.14, 141.61, 141.65, 176.38 ppm).
Pharmacological Evaluation
Compounds listed in Table 2 were evaluated in three now very well established classical tests for assessment of their antiepileptic potential in mice i.e. MES, ScMET and Rotorod tests. Each compound was administered orally to male NMRI mice (20-25g) as a suspension in a solution of tragacanth (1%). The dose-effect behavior of 15 tested products was examined by the administration of five different doses of each compound, treating ten mice at each dose. One hour after administration of the drug, the animals were submitted to the MES test. Maximal electroshock seizures were elicited with a current of 30 mA at 50 Hz delivered for 0.2 s via corneal electrodes. Mice were considered as protected if the tonic extension of hind limbs was not observed. The ED50 and the 95% confidence intervals were computed according to the method of Litchfield and Wilcoxon. The scMET and Rotorod ED50’s protocols were in all respects similar to those employed by the Anticonvulsant screening Project of the NIH (Bethesda, MD, USA). Compounds 3 was initially tested in phase I of the Antiepileptic Drug Development Program (Department of Health and Human Services, National Institutes of Health, Bethesda, MD, USA) and on single animals at 30, 100, 300 in the MES and sc MET test and was found active in the MES and scMET tests.
The authors wish to acknowledge the generous contribution of the CTB (Coopération Technique Belge, Brussels, Belgium) both in terms of fellowships (U.C K.) and financial support.