Clinical & Experimental Cardiology
Open Access
ISSN: 2155-9880
ISSN: 2155-9880
Research Article - (2022)Volume 13, Issue 2
Introduction: Carbohydrate antigen 19-9 (CA 19-9) is the most valuable as a serum tumor marker for pancreatic and biliary cancers. Different assays may show significant differences in individual patients or some samples. The aim of this paper is to explore the causes of differences in results from different instruments and to avoid them.
Methods: CA19-9 was detected using ARCHITECT i2000 analyzer. If the results of CA 19-9 on the ARCHITECT i2000 analyzer were over 39 U/mL, the serum specimens were secondly tested using Roche cobas e601 analyzer. The differences of CA19-9 were analyzed in samples between different instruments.
Results: The CA 19-9 concentrations tested using ARCHITECT i2000 assayer were significantly higher than that in cobas e601 assayer of all specimens, males and females (p=0.000, all). Of the 158 specimens, 106 specimens were inconsistent, and 52 specimens were consistent.
Conclusion: Gender and nonmalignant diseases were not the reasons induced the discordant results. If there is any clinical doubt about a result, CA 19-9 should be determined using another method to exclude possible interferences.
CA19-9; Different assays; Tumor markers; Automated immunoassays; Nonmalignant diseases
CA 19-9 is a glycolipid, the sialylated form of the Lewis blood group antigen. In serum, it exists as a mucin, a highmolecular-mass (200- 1,000 kd) glycoprotein complex. CA 19-9 is synthesized by normal human biliary ductular and pancreatic cells and by colon, gastric, endometrial, and salivary epithelia [1]. The original monoclonal antibody against CA 19-9 was developed from a human colon carcinoma cell line, SW-1116 [2].
Nowadays, CA 19-9 was mainly tested by commercially automated immunoassays. There are Abbott ARCHITECT iSystems,Beckman Coulter Access/UniCel DxI,Roche Elecsys/E170/cobas e Systems and Siemens ADVIA Centaur/Dimension Vista Systems. Although the CA 19-9 assay show acceptable analytic performance by different manufacturers, containing limit of detection, linearity, imprecision, method comparison and reference intervals, discrepancies of that are still commonly observed. Research has shown that the CA 19-9 assays give quite variable results especially at low and moderately elevated concentrations [3]. This study will analyzes the differences of CA19-9 in samples between different instruments.
Patients and CA 19-9 testing
CA 19-9 was tested in 5036 asymptomatic patients presented physical examination in Zhejiang Hospital. Empty stomach blood samples of all the patients were centrifuged at 3,000 rpm for 10 minutes and firstly tested for CA 19-9 using chemiluminescent microparticle enzyme immunoassays on the ARCHITECT i2000 analyzer (Abbott Diagnostics, Wiesbaden, Germany). If the results of CA 19-9 on the ARCHITECT i2000 analyzer were over 39 U/mL, the serum specimens were secondly tested using electrochemiluminescent immunoassays on the cobas e601 analyzer (Roche Diagnostic System, Basel, Switzerland). The reference intervals recommended by the manufacturers are 37 U/mL for ARCHITECT and 39 U/mL for Cobas, and we chose the 39 U/mL as cutoff value for further analysis. Samples were analyzed within 48 h and stored at 4℃ over 8 hours. During all Samples testing, the instruments are in a stable and controllable state. All patients were identified by physical examination number and reviewed the diseases from the physical examination reports.The study was approved by Ethics Committee at Zhejiang Hospital.
Statistical analysis
The CA 19-9 levels were expressed as the frequency, mean and median (quartile). The comparison of the CA 19-9 levels by the same analyser were calculated using two independent samples nonparametric test (the Mann-Whitney U test), and between two analysers the results were calculated using Paired sample Wilcoxon Signed Ranks test. The correlation of two CA 19-9 assays were accessed by Spearman rank correlation coefficient, and concordance was calculated using Passing-Bablok regression by MedCalc software. The relation between the diseases and the differences in two analysers were analysed by Logistic regression analysis. P<0.05 was considered to be statistically significant. Data were analysed with using SPSS 18.0 software.
Analyze the correlation and concordance of the two methods
158 specimens (3.14%) with elevated CA 19-9 levels were enrolled and stemmed from 5036 asymptomatic patients using ARCHITECT i2000 assayer. Of these, although the number of females was higher than that of males (female: male=111/47), results of CA19-9 in the two groups were no significant difference (p=0.129). The 158 specimens were further tested using cobas e601 assayer. 106 specimens were shown in normal level and only 52 specimens (32.91%) were shown in elevated level. Among the 52 concordant positive results between the two CA 19-9 assays, 43 specimens still have higher CA 19-9 levels in ARCHITECT i2000 assayer than that of in cobas e601 assayer. Of the 158 specimens, the CA 19-9 concentrations tested using ARCHITECT i2000 assayer were significantly higher than that of in cobas e601 assayer in all specimens, males and females (p=0.000, all) (Table 1).
| ARCHITECT i2000 | cobas e601 | |||||
|---|---|---|---|---|---|---|
| All | Male | Female | All | Male | Female | |
| n | 158 | 47 | 111 | 158 | 47 | 111 |
| Range | 39.01-1200.00 | 39.01-1200.0 | 39.17-1200.00 | 6.11-1000.00 | 6.11-309.70 | 10.25-1000.00 |
| Mean | 91.13 | 91.92 | 90.79 | 46.22 | 46.69 | 46.02 |
| Percentiles | ||||||
| 25th | 45.66 | 42.9 | 48.08 | 30.81 | 21.72 | 24.19 |
| Median | 55.66 b | 50.26 a,b | 57.00 b | 30.81 | 34.14 | 30.76 |
| 75th | 78.87 | 88.15 | 77.96 | 44.36 | 49.16 | 43.69 |
| Za | 1.518 | |||||
| Zb | 10.504 | 5.154 | 9.13 | |||
Note: a) Mann-Whitney U p=0.129 against female tested by ARCHITECT i2000, b) Wilcoxon Signed Ranks p=0.000 against the same group tested using cobas e601.
Table 1: Distribution of CA 19-9 concentrations (U/mL) tested using ARCHITECT i2000 and cobas e 601 in 158 apparently healthy individuals with elevated CA 19-9 levels using ARCHITECT i2000 assayer.
The values of CA19-9 in 158 patients were analyzed from two aspects, over 39 U/mL and 39 to 200 U/mL using ARCHITECT i2000 assayer. Method comparison demonstrated a poor agreement between ARCHITECT i2000 and Cobas e601 (Figure 1), with correlation coefficients of r=0.525 and r=0.508 (Spearman, P<0.0001).
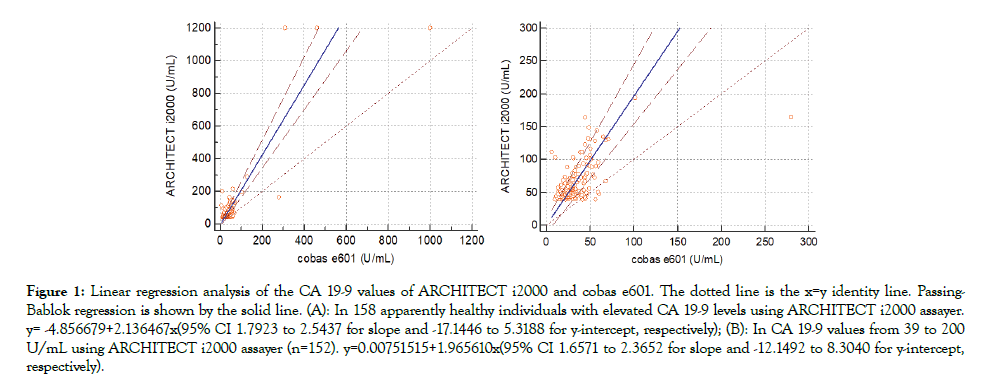
Figure 1: Linear regression analysis of the CA 19-9 values of ARCHITECT i2000 and cobas e601. The dotted line is the x=y identity line. Passing- Bablok regression is shown by the solid line. (A): In 158 apparently healthy individuals with elevated CA 19-9 levels using ARCHITECT i2000 assayer. y= -4.856679+2.136467x(95% CI 1.7923 to 2.5437 for slope and -17.1446 to 5.3188 for y-intercept, respectively); (B): In CA 19-9 values from 39 to 200 U/mL using ARCHITECT i2000 assayer (n=152). y=0.00751515+1.965610x(95% CI 1.6571 to 2.3652 for slope and -12.1492 to 8.3040 for y-intercept, respectively).
Gender was not the factor that cause differences
Of the 158 specimens, 106 specimens were inconsistent and 52 specimens were consistent. Chi-square test show different genders were not the reason induced the discordant results (Pearson Chi- Square=1.711,P=0.191) (Table 2).
| ARCHITECT i2000 > 39 U/mL | total | Pearson Chi-Square Value | P(2-sided) | ||
|---|---|---|---|---|---|
| cobas e601>39 U/mL | cobas e601 ≤ 39 U/mL | 1.711a | 0.191 | ||
| Male | 19 | 28 | 47 | ||
| Female | 33 | 78 | 111 | ||
| Total | 52 | 106 | 158 | ||
Note: a) 0 cells (.0%) have expected count less than 5. The minimum expected count is 15.47.
Table 2: The 158 specimens classified by gender.
Basic diseases cannot cause different results of two assays
The instruction of ARCHITECT CA 19-9XR is shown that 5.67% (25/441) samples from patients with nonmalignant disease were shown elevated CA 19-9 levels. In our study, the underlying diseases and nonmalignant diseases of the 158 individuals were reviewed from the physical examination reports. The quantitative value of elevated CA 19-9 levels using ARCHITECT i2000 with or without nonmalignant disease were shown in Table 3 and the differences were no significant. The frequency of the accordant and discordant results by the two CA 19-9 assays with or without nonmalignant diseases were shown in Table 3, and there were still no significant differences. Logistic regression had shown that nonmalignant diseases were not the reason induced the discordant results.
| i2000>39 U/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Underlying diseases N | e601 ≥ 39 U/mL(n=52) | e601 ≤ 39 U/mL(n=106) | Pearson chi-squarea | P valuea | OR | 95%IC | P valueb | ||
| Hypertension | |||||||||
| Yes | 24 | 11 | 13 | ||||||
| No | 134 | 41 | 93 | 2.14 | 0.143 | 0.491 | 0.181, 1.332 | 0.163 | |
| Diabetes | |||||||||
| Yes | 15 | 5 | 10 | ||||||
| No | 143 | 47 | 96 | 0.001 | 0.971 | 1.545 | 0.347, 6.881 | 0.568 | |
| Coronary heart disease | |||||||||
| Yes | 6 | 3 | 3 | ||||||
| No | 152 | 49 | 103 | 0.825 | 0.364 | 0.48 | 0.071, 3.261 | 0.453 | |
| hyperplasia of mammary glands cyclomastopathy | |||||||||
| Yes | 53 | 18 | 35 | ||||||
| No | 105 | 34 | 71 | 0.04 | 0.842 | 0.745 | 0.330, 1.683 | 0.479 | |
| Thyroid nodule | |||||||||
| Yes | 19 | 20 | 51 | ||||||
| No | 139 | 32 | 55 | 1.313 | 0.252 | 1.605 | 0.748, 3.446 | 0.225 | |
| Hysteromyoma | |||||||||
| Yes | 17 | 4 | 13 | ||||||
| No | 141 | 48 | 93 | 0.795 | 0.384 | 1.37 | 0.390, 4.806 | 0.623 | |
| Hepatic adipose infiltration | |||||||||
| Yes | 26 | 7 | 19 | ||||||
| No | 132 | 45 | 87 | 0.505 | 0.477 | 2.379 | 0.762, 7.432 | 0.136 | |
| Gall-stone, Gallbladder polyp | |||||||||
| Yes | 27 | 11 | 16 | ||||||
| No | 131 | 41 | 90 | 0.904 | 0.342 | 0.6 | 0.234, 1.536 | 0.287 | |
| Prostatic hyperplasia | |||||||||
| Yes | 19 | 7 | 12 | ||||||
| No | 139 | 45 | 94 | 0.151 | 0.697 | 1.288 | 0.340, 4.872 | 0.709 | |
| Obesity | |||||||||
| Yes | 28 | 12 | 16 | ||||||
| No | 130 | 40 | 90 | 1.525 | 0.217 | 0.472 | 0.168, 1.323 | 0.153 | |
a: each diseases analysed by Pearson chi-square test.
b: single factor logistic regression was used in the entry method.
Table 3: The frequency of the CA 19-9 using ARCHITECT i2000 and cobas e601 with or without underlying diseases.
The determination of CA19-9, as a tumor marker, is frequently test. Due to the high incidence of cancer, enhance health awareness of the early screening for tumors and the lack of specific tumor markers, CA19-9 was considered to be the one of the tumor markers for the routine physical examination of asymptomatic people in recent years [4]. But results obtained by different assays were varying and a change of method during follow-up may cause problems. Both Abbott ARCHITECT i2000 Systems and Roche cobas e601 Systems use the same monoclonal antibody (1116-NS- 19-9). The 2 assays give quite variable results especially at low and moderately elevated concentrations. For this interval, we analyze the differences between the instruments. It has been reported that age and gender affected the level of CA19-9. CA19-9 has different reference range in different age groups and which in female is higher than in male. So, it is recommended to set different reference ranges according to gender and age [5]. We analyzed 158 specimens and found that 106 specimens were inconsistent and 52 specimens were consistent. The cases of female (111 cases) is more than male (47 cases). The results of CA19-9 in male are higher than that in female in both the two instruments. There is no statistical difference of results between male and female in ARCHITECT i2000 Systems (p=0.129). This justifies the use of common reference limits for male and female [3]. The results of the two instruments were different between different genders groups (p=0.000), but gender was not the reason induced the discordant results (p=0.191).
CA19-9 is the most valuable as a serum tumor marker for pancreatic and biliary cancers, which is higher in liver, breast and gynaecological cancers. Even, elevated levels may also occur in benign diseases [6]. It has been shown that an increase in the CA 19-9 level in patients suffering from the gradual improvement of thyroid function and hypothyroidism resulted in resolution of the elevated CA 19-9 levels [7]. Recent reports have suggested that diabetes mellitus may be, in part, responsible for elevation of CA 19-9 [8,9]. But we found the differences of the quantitative value of elevated CA 19-9 levels using ARCHITECT i2000 with or without nonmalignant diseases (Hypertension, Diabetes and Coronary heart disease, et al) were no significant. The frequency of the accordant and discordant results by the two CA 19-9 assays with or without nonmalignant disease were shown in Table 3 and there were still no significant differences. Logistic regression has shown that nonmalignant diseases were not the reason induced the discordant results.
This study shows that results of CA19-9 were higher in Abbott’s than Roche. Other studies show that Abbott’s results were lower than Roche [3,10]. We consider that the reason is the inconsistent population. We chose the results of CA 19-9 on the ARCHITECT i2000 analyzer which were over 39 U/mL in relatively healthy population. Berth M found Rheumatoid factor interfered the determination of CA 19-9 [11]. The ADVIA Centaur system appears to be more sensitive to rheumatoid factor interference. Report shows that taking spirulina can cause elevated 19-9 levels in apparently healthy patients [12]. We speculate that there are two reasons for these discrepancies. First, this inconsistency is caused by a different instrumental methodology [13]. Second, ARCHITECT i2000 Systems appears to be more sensitive to some kinds of interferences than cobas e601. But this need further experiments.
Nowadays, there is still no reason to explain this inconsistency and no way to rule out it. Study recommends that patients always be monitored for CA19-9 with the same assay and that the method used should always be indicated in the reports [14]. If there is any clinical doubt about a result, CA 19-9 should be determined using another method to exclude possible interferences [11].
CA 19-9 was mainly tested by commercially automated immunoassays. There are Abbott ARCHITECT iSystems, Beckman Coulter Access/UniCel DxI, Roche Elecsys/E170/cobas e Systems and Siemens ADVIA Centaur/Dimension Vista Systems. Although the CA 19-9 assay show acceptable analytic performance by different manufacturers, containing limit of detection, linearity, imprecision, method comparison and reference intervals, discrepancies of that are still commonly observed. Gender and non-malignant diseases were not the reasons induced the discordant results. If there is any clinical doubt about a result, CA 19-9 should be determined using another method to exclude possible interferences. Research has shown that the CA 19-9 assays give quite variable results especially at low and moderately elevated concentrations. These studies will analyses the differences of CA19-9 in samples between different instruments.
We are granted to the patents who participated in this study for their time and generosity.
Conflict of interest
The authors declare no conflict of interests.
Citation: Li M, Xu P, Wang L, Shen S (2022) Study the Differences of Carbohydrate Antigen 19-9 Values in the Two Automatic Immune Analyzers. J Clin Exp Cardiolog.13: 715.
Received: 14-Feb-2022, Manuscript No. JCEC-22-14055; Editor assigned: 18-Feb-2022, Pre QC No. JCEC-22-14055 (PQ); Reviewed: 28-Feb-2022, QC No. JCEC-22-14055; Revised: 07-Mar-2022, Manuscript No. JCEC-22-14055 (R); Published: 14-Mar-2022 , DOI: 10.35248/2155-9880.22.13.715
Copyright: © 2022 Li M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.