
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Case Study - (2023)Volume 13, Issue 6
Paraquat Dichloride (PQ) poisoning could develop serious acute medical complications that have a high case fatality rate. There is inadequate evidence-based recommendation for the treatment, even with active treatment, the fatality rate is high. PQs are known to interfere with various cell signaling pathways, induce the formation of Reactive Oxygen Species (ROS), and ultimately cause cell damage. In the current study, we sought to provide treatment strategies in patients with PQ poisoning and then describe a case that consumed an enormous of PQ that successfully treated.
Paraquat dichloride; Poisoning; Immunosuppressants; Antioxidants
Understanding paraquat activity
Pesticides are compounds that are used to kill pests. They contained compounds labeled as insecticides, rodenticides, herbicides, fungicides, and fumigants [1]. Paraquat is a non- selective herbicide that belongs to the chemical family of bipyridyl quaternary ammonium salt. Oral ingestion is the most common pathway of poisoning. Paraquat is highly toxic to humans after oral ingestion. The initial clinical manifestation is mainly due to mitochondrial damage, Lipid peroxidation, and activation of Nuclear Factor Kappa B (NF-κB), generation of free radicals, decreased glutathione production, and increased inflammatory mediators like IL-1β, TNF-α, and IL-8. The clinical features of PQ poisoning can be classified into three types. Oral irritation and gastric upset due to mild poisoning and its prognosis is complete recovery. Moderate poisoning can cause acute renal failure, hepatitis followed by pulmonary fibrosis, and death after 2-3 weeks. Fulminant poisoning leads to multiple organ failure and cardiogenic shock resulting in death within hours to a few days after ingestion [2]. Paraquat takes up against a concentration gradient in lung tissue leading to respiratory function with pulmonary fibrosis. This herbicide also damages other organs leading to myocardial, renal, and hepatic failure, and pancreatic injury, which indicates a poor prognosis [3]. Paraquat is mainly excreted by the kidneys. Thus, the kidneys are one of the organs that accommodate the highest concentration of paraquat, which clarifies why acute renal injury happens in the early stage of paraquat poisoning [4]. However, due to the lack of effective therapies, the mortality rate of PQ poisoning during the period of 2001-2017 was in the range of 43%-71% in Iran [5-9], which is similar to the results of the other studies [10].
Pathophysiology/Mechanism of injury
PQ toxicity has two main relative consequences. The first is the generation of Reactive Oxygen Species (ROS), notably superoxide anions, hydrogen peroxide, and hydroxyl free radicals. They induce damage because they are highly reactive to cellular macromolecules. The second is coordinated with oxidation-reducing equivalents which are necessary for normal cell functioning, such as NADPH, an electron donor, and reduced Glutathione (GSH) [11]. Various protein/signaling pathways were reported about PQ-induced toxicity, such as Endoplasmic Reticulum (ER) induction, apoptosis, mitochondrial damage, and inflammation [12]. ER stress was mediated by pro- inflammatory mediator Toll-Like Receptor4 (TLR4), edema, and inflammation. This results in neutrophil infiltration and subsequent activation of pro-inflammatory cytokines, such as Interleukin-1β (IL-1β) and Tumor Necrosis Factor-Α (TNF-α). Cell apoptosis was produced by the up-regulation of the proapoptotic gene (Bax) and the down-regulation of the antiapoptotic gene (Bcl-2). Increasing the cleavage of caspase-8 (key mediators of extrinsic apoptosis) and Bid are responsible for cytotoxicity [13]. Furthermore, PQ triggers the mitochondrial function via the reduction of the adenosine triphosphate levels. This brought about active complexes I and IV and caused mitochondria toxicity via mitochondrial membrane swelling and depletion of cytochrome C [14].
Other studies also reported other molecular mechanisms related to PQ-induced toxicity, like the activation of Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2) signaling pathway, inhibition of Nuclear Factor Kappa B (NF-κB), and JNK/p38 Mitogen- Activated Protein Kinase (MAPK) signal pathway. Furthermore, PQ has induced Myeloperoxidase (MPO) an abundant proinflammatory enzyme found in neutrophil primary granules and monocyte lysosomes, Malondialdehyde (MDA) as a Lipid Peroxidation (LPO) marker. It attenuates the activity of antioxidant enzymes, including Superoxide Dismutase (SOD), Catalase (CAT), and Glutathione S-Transferases (GSTs). The clinical presentation of PQ intoxication is under these intracellular mechanisms [15].
Treatment options
We have discussed a few of the under investigation treatment options. No specific treatment or antidote is acknowledged for PQ intoxication. All patients of PQ intoxication should be treated timely within 10 hours after exposure or ingestion. There are three main aspects of treatment includes prevention of absorption, elimination of PQ from the body, and pathophysiological treatment. Absorption can be prevented by the feed of Fuller's earth (30% w/v), multiple charcoal activation, and sucralfate. Increasing urinary output can also aid in elimination. Drug interventions such as cyclophosphamide, methylprednisolone, pirfenidone, desferrioxamine, dexamethasone, and vitamins E, C, and D due to their immunomodulatory and antioxidant property have provided benefits. Some studies indicated specific treatments depending on the area of damage caused by paraquat, and we will discuss in the below content.
Initial management in ICU
Airway management and decontamination: Contrary to basic life-support measures, administering supplemental oxygen should be avoided because this can exacerbate PQ-mediated ROS production and subsequent lung damage [16]. Absorption can be prevented by the feed of Fuller's earth (30% w/v), multiple charcoal activation, and sucralfate. Junbo, et al. [17], results showed that early gastrointestinal lavage with sucralfate effectively improves signs and symptoms , reduces mortality rate and the cytokine levels (Transforming Growth Factor (TGF)-β1, Interleukin (IL)-10, and Tumor Necrosis Factor (TNF)-α), and improve the pathological injuries of the lungs and kidneys rats [17]. Early nasogastric tube insertion is recommended to maintain nutrition. Patients should not put anything in their mouth to avoid oral/oropharyngeal injury. These lesions due to this corrosive poison can be very painful and may require anticholinergic drugs such as diphenhydramine, Anesthetics such as lidocaine, and antacids, or mucosal coatings such as magnesium or aluminum hydroxide, kaolin, or sucralfate [18].
Hemodialysis and hemofiltration: The elimination of PQ could be increased due to its low molecular weight. Extracorporeal elimination might be valuable in the early phase, including hemodialysis and hemofiltration [19-22]. The 90-day survival rates of paraquat poisoning patients who received combined continuous venovenous hemofiltration and hemoperfusion treatment significantly improved [18]. Nevertheless, diverse studies show contrary results for all these modalities which can be caused by the high volume of distribution of PQ (1.2 L/kg -1.6 L/kg). Although, hemodialysis is used for the majority of poisoning patients, no significant relationship was found between hemodialysis and clinical outcome [23].
Prevention/Management of organ damage
Immunosuppressants: There are positive results from different studies that drove the immunosuppression therapy with methylprednisolone, Cyclophosphamide (CP), and Dexamethasone (DEX) in acute PQ poisoning. The work by Yen, et al. [24], on mice with paraquat-induced renal dysfunction established the combination of DEX and CP concurrently suppressed renal dysfunction, leukocyte or macrophage infiltration in the kidneys, induction of genes involved in inflammatory responses in the kidneys, and increased levels of specific lipid peroxidation markers in body fluids and in renal and pulmonary tissues, however, DEX only partially suppressed the macrophage infiltration [24].
The benefit of methylprednisolone and cyclophosphamide was also examined by Koh, et al. [25]. They worked on 85 patients over 2 years into additional immunosuppression with intravenous methylprednisolone and cyclophosphamide. Finally, immunosuppression therapy due to counteract immune-mediated inflammation, improves the survival of patients with admission eGFR<50 ml/min/1.73 m2 and WBC count>11,000/μL.
Immunosuppression therapy may play an important role in the survival of patients. Lin, et al. [26], work founded that on the repeated pulse of methylprednisolone (1 g/day for 3 days) and cyclophosphamide (15 mg/kg/day for 2 days) with continuous dexamethasone (20 mg/day) therapy for patients with severe paraquat poisoning until PaO2 was>80 mm Hg and if PaO2 was <60 mm Hg may need to repeat. In Wu, et al. [27], work, the result of 1811 PQ-poisoned people under treatment with hemoperfusion, in combination with cyclophosphamide, methylprednisolone, and dexamethasone was shown significantly raise survival rate (P=0.001).
A meta-analysis combining five studies (three Randomized Controlled Trial (RCTs)) in this field, demonstrated that the mortality of moderate to fulminant patients with PQ intoxication receiving the pulse therapy with glucocorticoid and cyclophosphamide was lower than that of the controls (P=0.0004). The treatment also reduced the mortality of patients with moderate to severe PQ intoxication (P=0.002), While, the therapy did not decrease the incidence of the ARF and hypoxia. Further, the pulse therapy induced more leucopenia than the controls (P<0.00001) [28].
Antioxidants: N-Acetylcystein (NAC) is capable of stimulating and producing glutathione and acting as a free radical scavenger. In vivo, a study has shown rats pretreated with NAC displayed less edema and cellular infiltration in the lung tissue and reduced the infiltration of inflammatory cells [11].
Desferrioxamine, as a chelating agent has been shown to reduce toxicity in PQ poisoning animal models. In the oxidative stress process, lipid peroxidation may be enhanced by iron radicals so desferrioxamine may have important role [29]. In another study, it was also followed by hemodialysis, and N acetyl cysteine was used in the management of a single patient with severe paraquat intoxication. In addition, in a study report in Korea desferrioxamine was a part of paraquat poisoning protocol [30].
Selenium (Se) protects against pro-oxidant-induced liver necrosis in Selenium deficient rodents. The research reported that repletion of cellular Glutathione Peroxidase (GPX1) activity followed by a single injection of Se. Therefore a review of this study, showed that the result, compared with the saline injection, pretreating the Se-deficient mice with the Se injection significantly reduced the mortality rate from 90% to 50% (p<0.05) in PQ poisoning, and prolonged their mean survival times of those eventually killed by paraquat toxicity from 8.8 to 54.3 h (p<0.05) [31].
Vitamin C as a scavenging free radicals agent due to confers protection by contributing an electron to reduce free radicals and neutralizing these compounds can regenerate other small antioxidant molecules, such as α-tocopherol, glutathione, and β carotene. In a study Vitamin C at a dose of 20 mg/kg body weight was given concomitantly with PQ to rats vitamin C administration restored PQ-induced morphopathological structure in liver and kidney tissues [32].
Melatonin (MEL), the pineal hormone, has strong neuroendocrine immunomodulatory activity and can scavenge free radicals to confer resistance to oxidation. Melatonin can up- regulated mitophagy and reduce the inflammatory response mediated by Toll-Like Receptor 9 (TLR9), the levels of TNF-α and IL-1β in the body [33]. The results generally showed that MEL could alleviate the infiltration of inflammatory cells and pulmonary fibrosis induced by PQ exposure [34].
Recent advances: Chloroquine (CQ) is known for lung protective effects in acute hemorrhagic necrotizing pancreatitis. Chloroquine also could rescue alveolar type II cells (A549 cells) from PQ-induced death. Based on the above findings, Shen, et al. [34], revealed that CQ could reduce lung injury due to paraquat in mice by altering inflammation, oxidative stress, and fibrosis [35].
Pirfenidone is a potentially anti fibrotic, analgesic, antipyretic, and anti-inflammatory agent. Its anti-fibrosis effects seem to be due to its antioxidant, anti-Transforming Growth Factor-Beta (anti-TGF-β) anti-Tumor Necrosis Factor (TNF)-alpha, up-regulation of Regulator of G-protein signaling 2 (RGS2). Lipid peroxidation antioxidant findings and histological analysis in lung tissue of male mice confirmed the anti-fibrotic effects [36]. Inhibition of TGF-β1 gene expression and production is also reported for pirfenidone in the bleomycin model of lung fibrosis in hamsters [37].Doxycycline has both anti-infection and matrix metalloproteinase inhibitory effects. Matrix metalloproteinases play an important role in the process of PQ-induced pulmonary fibrosis because they are capable of regulating chemokines and cytokines and so have an important role in the regulation of the inflammatory process and cell migration to the inflammatory site. Therefore in animal models, Doxycycline as Matrix metalloproteinase inhibitor, phenocopy alleviates paraquat- induced acute lung injury [38].
In rat module study by Zhang, et al. [38], demonstrated Rosiglitazone (10 mg/kg/d) was able to recover arterial oxygen partial pressure (PaO2), increase in the Wet-To-Dry (W/D) lung tissue weight ratio and lung fibrosis score by inhibition of reduction of protein and mRNA levels of PPAR-γ as inhibitor of Transforming Growth Factor-β1 (TGF-β1) expression and Phosphatase and Tensin Homolog (PTEN) as inhibitor of myofibroblast differentiation and prevent to elevate in protein and mRNA levels of TGF-β1.
Atorvastatin enhanced the paraquat-reduced cell viability and down-regulated the paraquat-induced expression of TLR-4 and nitric oxide production [39]. Moreover, another study mentioned the down-regulation of the Hypoxia-inducible factor HIF-1α/β‑catenin pathway may involve being considered as a therapeutic agent for PQ poisoning-induced pulmonary fibrosis [40]. Another statin, Simvastatin (SV), at a single oral dose of 20 mg significantly increased the GSH level and alleviated the severity of lung lesions due to its property to down-regulate the expression of inflammatory cytokines, decrease vascular leakage and inflammation in the lung injury. So, results demonstrated that statins pose a lung-protective effect on pulmonary injuries to PQ poisoning [41].
Febuxostat also has shown a protective effect against paraquat- induced lung toxicity in rats through inhibition of xanthine oxidase activity and oxidative stress, down-regulation of RAGE/ PI3K/Akt pathway, and suppression of β-catenin protein expression and its downstream inflammatory mediators [42].
Vitamin D treatments on PQ-induced lung fibrosis in mice are relieved through immunomodulatory actions of vitamin D on lung inflammatory diseases. Another study investigated vitamin D markedly suppressed the induction of TGF-β, Angiotensin II Type I Receptor (AT1R), α- smooth muscle actin SMA, collagen type I, and fibronectin and to the microvillus, in bleomycin- induced lung fibrosis in vitamin D deficient mice [43].
Lung damage is the most serious complication of PQ poisoning, but other organs such as the kidney, liver, and pancreas can also be affected by PQ. Gao Y, et al. [3], in rat models found that octreotide can antagonize pancreatic injury by significantly inhibition of the NF-κB signaling pathway and other inflammatory factors such as Tumor Necrosis Factor-alpha (TNF-α) and Interleukin-6 (IL-6), in the serum of acute pancreatitis models. In previous study, they found out the pancreas is damaged before the lungs and other critical organs in models with PQ intoxication which demonstrates a poor prognosis [3].
Report of chronic exposure to PQ
A 23-year-old Iranian female with no past medical history was brought to the Emergency Room (ER) of a hospital in northern Iran by her family, with symptoms of nausea and vomiting. She consumed 200 cc paraquat diluted with water 30 min before admission to commit suicide. At the very beginning, gastric lavage was performed for the patient, which reported green sticky secretions on it. The patient was subsequently transferred to be referred to the poisoning emergency department. After admission, physical examination showed that the patient’s vital signs were stable and that her oral mucosa was eroded. Cardiopulmonary function was normal. The laboratory examination results are shown in Tables 1a and 1b. She was transferred to the Intensive Care Unit (ICU) for special care from the perspective of respiratory symptoms; a decrease in oxygen saturation and preparation for hemodialysis. Hemodialysis was performed 3 times; 6, 4, and 4 hours (a total of 14 hours) in 3 days. Immunosuppressant, organ protection, and antioxidation drugs were administered. After admission, chest computed tomography showed localized alveolar infiltrate and involvement of the left lung due to aspiration, which is shown in Figure 1.
| Date | PH | Pco2 | Po2 | FIO2 | HCO3 | BS | Blood urea | Creatinine | PT | INR | Plc | WBC | NET (%) | Hgb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 06 May | 7.51 | 27.2 | 118.6 | 21 | 24.3 | 110 | 28 | 0.6 | 12.3 | 94 | 9.9 | |||
| 07 May | 7.54 | 29.7 | 115 | 21 | 25.6 | 111 | 15 | 0.9 | 19.3 | 1.87 | 166 | 12.3 | 84.6 | 9.1 |
| 08 May | 7.46 | 30.7 | 138 | 21 | 22.1 | 229 | 35 | 1.7 | 13.9 | 1.11 | 129 | 14.9 | 91.8 | 8.6 |
| 09 May | 7.45 | 36.3 | 69.6 | 21 | 26 | 186 | 39 | 1.8 | 14.2 | 1.14 | 44 | 8.5 | 96 | 8.1 |
| 10 May | 144 | 65 | 1.7 | 15.1 | 1.2 | 142 | 4.8 | 87.3 | 7.3 | |||||
| 11 May | 123 | 80 | 1.5 | 15.1 | 1.27 | 131 | 4.3 | 81.2 | 7 | |||||
| 12 May | 7.39 | 119 | 69 | 1.4 | 13.8 | 1.1 | 148 | 5.4 | 85 | 7.3 | ||||
| 13 May | - | 169 | 4.2 | 97 | 7.2 | |||||||||
| 18 May | - | 101 | - | 1.8 | 29.3 | 7.1 | ||||||||
| 19 May | 7.46 | 40.7 | 54.8 | 21 | 28.5 | - | ||||||||
| 20 May | - | 13.5 | 1.06 | 313 | 2.8 | 45.3 | 7.4 | |||||||
| 21 May | - | 4.4 | - | 8.1 | ||||||||||
Table 1a: Medical data table for patient monitoring.
| Date | Na | K | P | Ca | Mg |
|---|---|---|---|---|---|
| 07 May | 135.2 | 3.29 | |||
| 08 May | 3.1 | 9.2 | 1.95 | ||
| 10 May | 8.7 | 2.2 | |||
| 11 May | 144 | 3 | 2.9 | 8.5 | 1.8 |
| 12 May | 141 | 3 | 2.7 | 9 | 1.7 |
| 13 May | 137 | 4.1 | |||
| 14 May | 135 | 4 | |||
| 15 May | 132 | 4.6 | 9.2 | 1.7 |
Table 1b: Laboratory examination of the electrolyte levels of the patient.
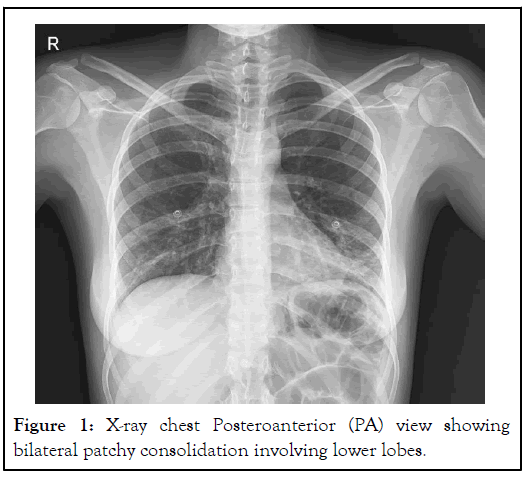
Figure 1: X-ray chest Posteroanterior (PA) view showing bilateral patchy consolidation involving lower lobes.
The patient received Methylprednisolone IV infusion via 500 mg over 2 hours BID for 3 days and then with Dexamethasone 4 mg every 8 hours was tapered. After obtaining consent and noting the side effects of cyclophosphamide, 1 g diluted in 500 cc Normal Saline (NS) daily was started on the second day for 3 doses. Mesna (sodium 2-mercaptoethane sulfonate) was also prescribed to the patient in order to prevent hemorrhagic cystitis complications of cyclophosphamide after consultation with the oncology service. Amp deferoxamine (500 mg every 12 hours), N-acetyl cysteine (8 g infusion over 8 hours and repeated every 8 hours), vitamin C (1 g every 8 hours) and E, selenium (500 µg daily), pearl CoQ10 (100 mg every 12 hours) also administered for 10 days. If the patient's platelet count was more than 70,000, Partial Thromboplastin Time (PTT) and International Normalized Ratio (INR) were less than 70 and 1.8 respectively; heparin ampoule was given 5,000 units twice a day. Pirfenidone at a dose of 200 mg three times a day was started on the 2nd day of the patient's admission. The patient also received a series of palliative treatments because, she had burning pain in the sternum and epigastria, which is most likely due to mucositis involving the esophagus and stomach (Figure 2), and throat areas and helps swallowing easier. For this purpose, she has prescribed a cocktail of diphenhydramine, magnesium hydroxide, tetracycline, dexamethasone, and lidocaine. Triadent gel® (Triamcinolone Acetonide) and Mucozamine spray® were prescribed to prevent the development of pseudomembranous mucositis on the tongue and mouth. Triadent gel was very effective, but the patient was not satisfied with Mucosamine® spray due to the formation of adhesion in that area. Sucralfate and pantoprazole were also prescribed to the patient to relieve stomach pain. The patient received Total Parenteral Nutrition (TPN) for 24 hours due to her inability to eat orally. Mild Acute Kidney Injury (AKI) was also detected with an elevation of SCr (1.7 mg/dL on the third day and 1.8 mg/dL on the fourth day) but she did not receive dialysis because of this, and finally it returned to normal after 6 days. The patient also complained of ear pain, which we requested for ENT service consultation, but it was not observed in the CT scan, and it seems that the pain was referred from a sore throat. Moreover, the patient had sialora caused by a swallowing disorder. 10 days after receiving cyclophosphamide, the patient's WBC dropped to 1300 cell/µl, and her neutrophil count was 500 cells/µl and got neutropenia so she received one dose of the G-CSF and after requesting infectious consultation, ceftriaxone and fluconazole were considered for her. Finally, 15 days after PQ consuming, she was discharged without any complications, including normal renal function (0.6 mg/dL of SCr), CXR without lung fibrosis, no hypoxia 98% of SaO2, and ALT of 13 U/L she was discharged in good condition, with no need for home oxygen therapy. Approximately after 2 weeks of treatment with cyclophosphamide, the patient’s hair loss started. 3 days after discharge the patient developed sputum with a blood streak for which amoxiclave in addition prednisolone until 10 days was started. Perfenidone was continued for 28 days. During the 3 months that the patient was followed up, the patient's general condition and anemia improved day by day, and she had no breathing problems, and she had a normal chest X-ray and spirometry and her hemoglobin reached 9.4 without the use of iron supplements (Figure 3).
Figure 2: Clinical photograph showing extensive oral ulcerations.
Figure 3: Computed Tomography (CT) scan of the lung.
In the present case of corrosive oral also known as ‘paraquat tongue’ is seen within the first few days of poisoning and entire gastrointestinal tract mucositis (epigastria comfort) along with features of hoarseness, sialora (due to Inability to swallow), However, the patient did not develop fever, short of breaths, decreased oxygen saturation level, tachypnea, and respiratory distress. The basis of treatment for this case was reducing exposure; enhancing elimination and using anti-inflammatory drugs and antioxidants. In current practice, the therapeutic regimen mainly consists of immunosuppressive agents, Cyclophosphamide (CP), Methylprednisolone (MP) and pirfenidone, and antioxidants. The use of CP and MP pulse therapy comes from the experience of treating patients with severe lung injury secondary to systemic lupus erythematosus [44], and about perfenidone due to good outcomes from COVID 19 treatments [45-48].
Paraquat can develop a serious acute conditions and complications, including acute respiratory distress syndrome, progressive lung fibrosis, renal injury, cardiovascular collapse, and liver and neurotoxicity. Also, studies have shown that chronic exposure to PQ increases the risk of Parkinson's disease. Moreover, the emergency rescue stage is critical in treating paraquat poisoning and seeking timely and effective clinical treatment can reduce the mortality rate. Therefore, it is necessary to valuation research outcomes on acute paraquat poisoning and to implement well-timed and effective treatment. Although, paraquat poisoning is followed by oral ingestion, even with active treatment, the fatality rate is as high as 50%-70%. No specific antidote is available for this poisoning, and there is also inadequate evidence-based recommendation for the treatment. The management is mainly supportive. Immunosuppressive agents such as cyclophosphamide, methylprednisolone, and dexamethasone, along with antioxidants such as N-acetyl- cysteine and ascorbic acid, could be tried.
It is worth mentioning that all authors met the criteria for authorship by the recommendations of the International Committee of Medical Journal Editors.
The authors declare no conflicts of interest.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Khosravi N, Talabaki H (2023) Strategies for Enduring Severe Paraquat Consumption: Case Report and a Literature Review. J Clin Toxicol. 13:552.
Received: 04-Sep-2023, Manuscript No. JCT-23-26490; Editor assigned: 06-Sep-2023, Pre QC No. JCT-23-26490 (PQ); Reviewed: 20-Sep-2023, QC No. JCT-23-26490; Revised: 27-Sep-2023, Manuscript No. JCT-23-26490 (R); Published: 04-Oct-2023 , DOI: 10.35248/2161-0495.23.13.552
Copyright: © 2023 Khosravi N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.