Journal of Biomedical Engineering and Medical Devices
Open Access
ISSN: 2475-7586
ISSN: 2475-7586
Research Article - (2016) Volume 1, Issue 1
Keywords: 25-hydroxyvitamin D; IRMA; Displacement buffer; Radio iodination; Displacement buffer.
Vitamin D is a steroid hormone involved in the active intestinal absorption of calcium and regulation of calcium homeostasis. It is essential for the formation and maintenance of strong, healthy bones [6]. Vitamin D has other roles in the body, including modulation of cell growth, neuromuscular and immune function, and reduction of inflammation [7, 8]. Vitamin D has two isomers: vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol). Vitamin D2 is obtained from dairy product whereas Vitamin D3 is produced in the skin after exposure to ultraviolet light. In the liver Vitamin D is hydroxylated at its carbon 25 to form 25(OH) D. This metabolite is the predominant circulating form of vitamin D and is considered to be an accurate indicator of the general Vitamin D status on an individual [9-11].
Due to the high clinical importance of 25(OH) D a large number of methods are known from the literature. Which allow 25(OH) D to be more or less reliably determined the IRMA or noncompetitive reagentexcess, are distinguished from conventional radioimmunoassay (RIA) by use of purified radioactively labeled antibody instead of labeled antigen. IRMA can provide improved sensitivity and specificity. However, IRMA present some practical problems with nonspecific binding, increased consumption of antibody, biphasic dose response curve, (high dose hook effect).
Committee of the Institute of Medicine concluded that persons are at risk of vitamin D deficiency at serum 25(OH)D concentrations <30 nmol/L (12 ng/ml). Some are potentially at risk for inadequacy at level ranging from 30-50 nmol/L (12-20 ng/ml) [7].
Materials
Study population and serum sample collection. Serum were obtained from normal healthy volunteers (19 males and 11 females, aged between 28 to 45 years ;(N=30) and women patients (N=517). Blood samples were collected, serum were separated, and serum specimens were stored at -70°C until testing. Polystyrenes tubes 5 ml, 75 × 12 mm (article No.55.476.005Sarstedt AG & Co.) were coated with an house rabbit polyclonal antibodies anti 25-(OH)D raised in four females rabbits (12 weeks aged) against 25hydroxy vitamin D-3- hemisuccinate conjugated to bovine serum albumin (BIOCODE/hycel Liège , Belgium) Reagents 5 mCi iodine-125, (AEC-Amersham soc Ltd), monoclonal Antibody that recognizes both 25-OH Vitamin D3 (Sparkasse Aachen) and, 25(OH)D”( Sparkasse Aachen) and 25(OH) D2 (Sparkasse Aachen); Vit D Displacement buffer: 1.6 g/L 8- anilino-1-naphthalenesulfonic acid (Sigma-aldrich, St. Louis, Mo)mix with 160ml ethylene glycol (Sigma-aldrich, St Louis, Mo). Carbonatebicarbonate buffer capsules R36/3/38; S26-36 (Sigma-C-3041; Lot 107H8201); Synthetic 25-OH vitamin D peptide (ABIN 1047959, Aachen, Germany) stand stock were gravimetrically prepared for calibration: 3 μg dissolved in 100 ml anhydrous ethanol.
Preparation of anti-vitamin D coated tubes The adsorption was carried out as describe by Murthy and Moudgal [12] Goat anti-(rabbit IgG) serum and rabbit anti-vitamin D were both purified by ammonium sulphate precipitation to give the corresponding IgG fractions. Tube(polystyrene) were coated with goat anti(rabbitIgG), a purified IgG, 200 ul per tube of 20 mg/l IgG in 10 mM phosphate buffered saline (PBS) and incubated overnight at room temperature. The coated tubes were washed three times with 10 mM phosphate buffered saline, 0.05 tween 20(PBST). Rabbit anti-vitamin IgG, 0.05 sodium azide, 150 ul per tube was added and tubes stored at 2-8*C.
Vitamin D displacement agent By displacement is meant full or partial separation of some or all of the vitamin D metabolite from the factor to which it is bound in the serum. Preferred agents for use in the present work are chemical reagents (1.6 g/L 8-anilino-1- naphthalenesulfonic acid, ethylene glycol and methanol [14] which act by disruption or destroying the bond between 25(OH) D and binding factor.
Radioiodination of monoclonal 25(OH) D antibodies
A monoclonal 25(OH) D is labeled with radioisotope 125-iodine by a direct labeling method by exhaustive chloramine-T to obtain a radioisotope MAb-25(OH) D. [14]. Briefly: The monoclonal 25(OH)D antibody was radiolabeled using exhaustive chloramine-T oxidation using sodium iodide-125 (185 MBq/ml). Three mmole/L of monoclonal antibody was mixed with 0.5 mCi/ 10 μl and 10 μl of 0.12 mmole/L of chloramines-T. The reaction was allowed to proceed for 3 minutes. The reaction is stopped with excess sodium metabisulfite. The three component of the radio labeling procedure (undamaged and damaged antigen and unreacted isotope) were separated by gel filtration chromatography using PD-10 G-25 sephadex [15].
Sandwich immuno radio metric assay
The serum sample is treated prior to analysis with mix ANS/ Ethylene glycol/Methanol in such a way that all serum protein which may bind 25(OH)D are broken down. The amount of 25(OH)D in the serum is determine using uncompetition binding analysis solid phase immune radio metric assay (IRMA). It is based upon, non-competitive displacement agents which enable effective separation of 25(OH)D from VDBP to enable the amount of 25(OH)D to be measured, without completing the 25(OH)D or requiring its solvent extraction from de serum.
All determinations were performed in duplicate and all step carried out at room temperature. Briefly: 25 μl of serum is diluted with 1 ml of displacement buffer (mix of 8-anilino-1-naphthalene sulfonic acid ammonium salt 1,6 g/L and ethylene glycol 160 ml/L) and standing the tube for 1 hr at room temperature to release the analyte from the protein bound. 100 μl of extract sample was pipette into the coated tube and pre incubated for 1 hr on a shaker at 150 rpm. The tube was washed with 10 mM phosphate buffered saline containing 0.05% Tween20(PBST) and followed by addition of radio labeled monoclonal anti-vitamin D and mix by shaking the test tubes rack gently and incubate for 2 hrs on a shaker at 150 rpm. After, decant all tubes, except Total count tubes (Total radioactivity) by simultaneous inversion with a sponge rack into a radioactive waste receptacle. Count all tubes in a gamma counter WALLAC LKB for one minute.
The curves of the diluted samples were parallel to the calibration curve: demonstrating that human 25(OH)D (in serum samples) and 25(OH)D (in the calibrator) had the same ability to displace the signal in the RIA.
The curve was done manually using log-log graph paper by plotting the known concentration of standards versus count per minute (cpm) . Plot a curve of % Bound (B) /Total activity (T) for each Standard (yaxis) against the concentration (x-axis) on log-log graph paper. Based on the % B/T for each control and unknown, determine the 25 (OH)D concentrations of the means of the duplicate counts of each control and unknown from the standard curve. (Interpolate the concentration of the sample from this standard curve) [16].
547 ”healthy, ambulatory Congolese women” (between 21 and 45 years old at the time of sample collection) showed that 427(78%) were deficiency are at level 25(OH)D < 14 ng/ml and 93 (17%) are sufficient at level 25(OH)D >14 ng/ml; 2 (0.3%) women were 25(OH) >51 ng/ml and 25 (4,5%) women were 25(OH)D <5 ng/ml.
Linear Regression Analysis of serum 25(OH)D values obtained with commercially available RIA kit. (a,b: constants in linear regression equation y=a × +b)
Normal ranges should be established by each laboratory using normal subjects. Results of normal range studies on the “cgeacrenk25 (OH)D solid-phase IRMA” are reported below.
Performance characteristics
Precision: Within-run and between-days variance was determined by analyzing four different whole-blood control samples (2.5, 7.3, 12 and 20 ng/ml) in duplicate over 14 days, two analytical runs per day, with a minimum of 2 hrs between runs.
Standard deviation calculated as: d=difference between duplicates, and n=no. duplicate determinations (shown in parentheses) 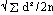
Linearity (Table 4) the serial dilution linearity of three serum samples is presented in Table 3. The relation between concentration and dilution of the serum did not significantly deviate from linearity of the concentration range studies. The overall recovery for the serial dilutions ranged between 87% and 109%.
| Normal ranges | N | Mean(ng/ml) | Range(ng/ml) |
| 30 | 15 | Nov-25 |
Table 1: Our laboratory reference range Our assay was linear at 11-25 ng/ml
| POOL 1 | POOL2 | POOL3 | POOL4 | |
|---|---|---|---|---|
| Wthin-run | ||||
| Mean +SD | 2.5 +0.5 | 7.3+0.7 | 12+ 1.3 | 20+ 1.3 |
| CV | 8.3 | 5.6 | 10.8 | 13 |
| Between-days | ||||
| Mean +SD | 2.3+0.6 | 6.8+1.0 | 24.6+2.2 | 24.6+2.2 |
| CV | 9.5 | 7.4 | 12.3 | 15 |
Table 2: Precision and reproducibility: Means, standard deviation (SD) in ng/ml, coefficients of variation (CV) in %.
| Within-batch (n=4) | Between-batch (n=6) | ||
|---|---|---|---|
| Extracted 25(OH)D | SD | Extracted 25(OH)D | SD |
| <7.6 | 0.5(8) | 5.1 | 0.6 |
| 7.7–15.4 | 0.7(6) | 10.8 | 0.7 |
| >15 | 1.3(10) | 23.9 | 1.7 |
Table 3: Precision of solid-phase immunoradiometric assay.
| Sample | Dilution | Expected | Observed | Recovery% |
|---|---|---|---|---|
| Factor | Cpm | Cpm | ||
| 1 | 3 | 14576 | 13266 | 91 |
| 4 | 7288 | 6713 | 92 | |
| 8 | 3644 | 3721 | 102 | |
| 16 | 1822 | 1913 | 104 | |
| 2 | 2 | 33321 | 29897 | 89.7 |
| 4 | 16660 | 16485 | 98.9 | |
| 8 | 8330 | 8442 | 101.3 | |
| 16 | 4165 | 4201 | 100.8 | |
| 3 | 2 | 60175 | 55397 | 92 |
| 4 | 30087 | 26798 | 89 | |
| 8 | 15043 | 14448 | 96 | |
| 16 | 7521 | 7924 | 105 | |
Table 4: The serial linearity of three serums.
Cpm=count per minute.
One obstacle to development of successful assays for vitamin D has been the technical difficulty in the isolation of tightly bound 25- hydroxyvitamin D3 and 25-hydroxyvitamin D2 from their vitamin D binding protein (DBP) in test biological samples. DBP is a serum glycoprotein that binds vitamin D sterols, G-actin, fatty acids and chemotactic agents [19].
Approximately 80% of 25(OH)D is bound to vitamin D binding protein (VDBP), 15% to albumin, and 0.03% free. The presence of DBP, presents a major difficulty in measuring serum levels of 25-(OH)D for this reason it is important to separate from vitamin D binding protein. On the other hand, non-extracted immunoassay method may be susceptible to matrix effects, particularly due to the lipophilic nature of 25(OH) D. taken together with the low (Nano molar) levels of vitamin D metabolites in serum, these factors have made the routine measurement b of 25(OH)D an analytical challenge [20].
Conventional laboratory technique for the determination of 25(OH)D in serum is very laborious. The 25(OH)D first have to be extracted from serum using organic solvents, and then purified by chromatography [21]. However, the disadvantages of HPLC quantification methods include their requirements for expensive equipment, large sample volumes, and technical expertise to perform this type of analysis [24]. Liquid chromatography-tandem mass spectrometry was recently proposed as a reference method for vitamin D status evaluation [19].
Using the obtained homemade polyclonal antibodies [25] and radioisotope iodine-125 labeled compound, we have established an IRMA system, in this work. RIA is the earliest of the immunoassay techniques. In RIA, the marker is an isotope of a radioactive element, hence the name radioimmunoassay. RIA is an extremely powerful tool. One of its main advantages is the sensitivity that can be achieved. Drug levels in serum and urine that are as low as 10 to 100 parts per billion are routinely measured. It is used as a diagnostic tool to detect and quantify numerous naturally occurring chemicals in human serum and urine. Another characteristic that makes RIA such a powerful tool is the specificity of the assay. The antibodies are highly specific for the drugs analyzed and they rarely make a mistake in recognizing the lockand- key fit between antibody and drug. One of the major limitations of the RIA is that it generates radioactive waste. Because of a short halflife, the majority is RIAs labeled with iodine 125; they have shelf life of only approximately sixty days [26].
The 25(OH)D IRMA described here in yielded consistent results over 2 months of assays by four operators. A significant number of Congolese women (between 21 and 45 years old at the time of blood serum collection) showed that 427 (78%) were deficiency at level 25(OH)D<14 NG/ML AND 93 (17%) are sufficient at level 25(OH)D >14 ng/ml; and 2 (0.3%) women were 25(OH)>51 ng/ml. Our clinical results with this assay are consistent with that reported by many group [27]. The 25(OH)D IRMA reported here is an accurate, precise and reliable manual method for measuring 25(OH)D concentrations in human serum. This method yields acceptably low CVs of <12% across the measurement range of the assay with good dilution linearity and analytical recovery characterization [28,29].
This study was limited in that most of the participants were women. As vitamin D deficiency is even more prevalent in men of African descent, and this population has a higher prostate cancer risk [30]. This study need to be replicate in future research and conducted in men, would be helpful to see of our results are generalizable.
But the use requires further evaluation, by clinical studies, before being considered as an alternative method in DRC determining Vitamin D status.
Measurement of 25(OH) D directly in serum, by IRMA will remain the method in our laboratory. Our kit called “cgeacrenk-25(OH)Dsolid-phaseIRMA” provide more suited to routine use in clinical biochemical laboratories and thus monitoring progression, regression, or stabilization of vitamin D deficiency in our population.
The management and successful therapy of all diseases in Democratic Republic of Congo, strongly depend on easy-to-perform, cost-efficient and reliable diagnosis. In order to improve and broaden patient access by reliable and cost-efficient diagnosis and to scale-up controlled treatment, so called “adapted technologies” are required, especially for point-of-care use in remote and rural areas.
We acknowledge our institution: “General Atomic Energy Commission” for having given us the opportunity to work and with financial support from Ir. Alphonse Tshiband head service RIA/kits productions; Dr. Ingala and Dr. Osongo.