Lupus: Open Access
Open Access
ISSN: 2684-1630
ISSN: 2684-1630
Expert Review - (2019)Volume 4, Issue 2
The most popular diagnostic criteria for SLE is the American College of Rheumatology (ACR) criteria, which does not often help in diagnosis due to several pitfalls, especially when it presents with haematological manifestations, which is confirmed by our observations over more than three decades and our studies on SLE. The problems and challenges in diagnosis are illustrated by the case histories and our own studies described in this article. The diagnosis of SLE is often delayed or even missed when patients come with haematological manifestations. The problem is even more serious when the index of clinical suspicion is low, especially in a situation where patients go doctor-shopping with improper follow up, which is common without a strong primary care set up and a referral system which are essential for streamlining patient care. Our first study had shown that majority of the patients had haematological manifestations at initial presentation. But the ACR criteria, which we currently use for diagnosis, do not give any importance to haematological manifestations. To compound that further, autoimmune hypothyroidism is also not included in the ACR criteria, in spite of it being a very common coexisting abnormality in these patients. The commonest haematological abnormalities at presentation were ITP, followed by autoimmune haemolysis and APLAS. Interestingly those with haematological manifestations often did not have any rheumatic complaints. It thus appeared to us that SLE is more of a haematological disorder rather than a rheumatologic disorder. A significant number of our patients did not satisfy the ACR criteria at the time of diagnosis but did so only on prolonged follow up. Thus the present criteria cannot help us to diagnose SLE presenting with haematological problem or other atypical manifestations and therefore needs an alternative. We have developed the “Kozhikode Criteria for SLE” to circumvent this lacunae. The second study was to validate the new criteria. The outcome of both these studies and on how to make diagnosis and management of SLE easy will be discussed in this article based on the case histories, my personal observations and our original studies. Those with SLE, just like any other chronic disease, were found to have abnormalities in diet and lifestyle, the modification of which could prevent the development of this diseases or modify the course after developing it.
SLE; ITP; Anaemia; APLAS; Kozhikode criteria; Lifestyle; ACR criteria
Presenting as Alopecia Areata with B12 deficiency
30 year old female was referred for evaluation of refractory anaemia, not responding to haematinics. On examination she had all the clinical features of B12 deficiency (knuckle pigmentation) and alopecia aerate (Figure 10).
Figure 10. Showing alopecia aerate.
Her brother had alopecia totalis and B12 deficiency. ANA was positive and Anti Ds DNA was borderline positive. She improved on Azathioprine and steroid, and is on follow up. This case history suggests that any autoimmune disorder known to us, can be a manifestation of SLE. It also suggests that autoimmunity can run in families, but is often not due to genetic predisposition. If people share the same diet, lifestyle and environment, they will have similar health status or disease status, it needs only common sense to arrive at this simple logic. Therefore in any disease, rather than blaming the gene, we should concentrate on improving the basic issues related to diet, lifestyle and environment.
The two studies and the case histories clearly indicate that majority of the presenting manifestations in SLE are haematological. SLE which was once considered an incurable disease has become almost curable now with effective therapy combined with modification of their diet and lifestyles. But that requires prompt clinical diagnosis and clinical recognition of the complications and comorbidities, with timely and appropriate interventions. Clinical skill is the corner stone, even today, for facilitating diagnosis and treatment of such patients. Relying too much on laboratory alone for the confirmation of SLE or its complications like cerebral vein thrombosis, might adversely affect their outcome. Lifestyle and diet modification has a role in preventing SLE and for better outcome after its development. Too much of compartmentalization and focus on protocol-based treatment alone will not be appropriate for better patient outcomes.
In both the studies, it was found that haematological manifestations were the commonest symptom altogether and also was the commonest presenting symptom. The findings demonstrated that haematological manifestations are a common presenting symptom and may be missed if the index of suspicion is low. This observation was contradictory to the description in western books [11,12]. There are some studies which have evaluated the haematological findings in SLE and the prevalence rates in them are comparable to our observations [13-16]. But haematological manifestations as the initial presentation and its improper inclusion in the criteria for diagnosing SLE were not addressed in any of these studies. The observation of inverse relation between the presence of musculoskeletal symptoms and haematological manifestations suggests that it is wrong to look for joint symptoms to suspect SLE. The commonly co existing autoimmune hypothyroidism in those presenting with haematological manifestations is under represented in the ACR criteria.
The subjects in the studies had a clinical diagnosis of possible SLE or evolving SLE at the time of initial presentation itself. Several ANA negative patients could be diagnosed only with application of the Kozhikode criteria, since they did not satisfy the ACR criteria [1,3]. ANA negative status cannot rule out SLE in its early stages. One study by Mc Hardy et al in 1982, had suggested 8.9% prevalence of ANA negative SLE [17]. Two other studies also found a prevalence of ANA negative SLE to be 5% [18,19]. Therefore it is important to rely more on clinical judgment rather than on the existing criteria alone for managing the disease. The studies and our observations emphasize the need for regular follow up of all suspected cases, even if they are ANA negative. To address the issues of early diagnosis, this author proposed the new criteria-The Kozhikode Criteria, which we have been using for the past two decades [1-3].
The second study was aimed at validating the Kozhikode criteria, which has shown the utility of it for early diagnosis, and its superiority over the ACR criteria. We could diagnose all the ANA negative SLEs only by applying the Kozhikode criteria. The second study has thus validated the Kozhikode Criteria as a useful tool for diagnosis of SLE.
In both studies we studied the diet and lifestyle of the patients. Patients with SLE were found to follow a sedentary lifestyle and not doing any kind of physical activity on a regular basis [1,3]. The physical activity levels in the newly diagnosed cases showed statistically significant decrease in comparison to the controls [1-3]. There were some studies establishing contribution of sedentary life style increasing the mortality in SLE, there were no studies relating them to the aetiology [20,21]. The dietary habit in patients is erratic with very poor intake of pulses, vegetables and fruits. Instead they were found to use fast foods, junk foods, fried meat and canned juices more often than controls, with a significant p value. These tow observations throw light on the aetiology of SLE and other autoimmune disorders. They all appear to be the end result of wrong diet and lifestyle where they get exposed to several unwanted substances and miss the protectives elements, including adequate exercise. The two studies clearly had shown the pit falls of the ACR criteria. With ACR criteria, SLE can be diagnosed only after more than one organ has been involved. Usually it will take years for patients to satisfy 4 out of the 11 criteria, and this results in delay in diagnosis and initiation of appropriate treatment. Haematological manifestations are seriously underrepresented in the ACR criteria. It may be that our subset of population only may be having higher prevalence of haematological manifestations, due to the differences in diet, life style and ethnicity. But if an attempt is made it could be so in other places too. However we can conclude that the ACR criteria, which was validated only on the western population, cannot be applied to Indian population.
Why SLE and Autoimmunity?
We have a long list of immunologic derangements identified in SLE. We study the receptors, genes, antibodies, and cytokines and consider them as aetiological factors but forget to see the macroscopic issues in diet and lifestyle. Such an approach will not help us in devising strategies for prevention of the disease. One mechanism suggested for the production of auto antibodies is macrophage dysfunction with defective uptake of apoptotic and necrotic cells whenever there is tissue damage by any mechanism. This is said to lead to autoantibody production; But the question is what decides macrophage function? Macrophage function depends on several factors including Vitamin D [8,9]. Vitamin D deficiency had been linked to severe manifestations in SLE [8]. The patients with SLE consumed very little vegetables, fruits and pulses, and they all preferred fried fish, fried meat and lots of junk food and fast foods. It is also observed that SLE is not seen in strict vegetarians who take a balanced diet and was more common in some communities which resort to junk foods and fried foods and no vegetables and fruits. Thus it is obvious that SLE/ITP is only a manifestation, and the most important investigation to find out aetiology is clinical skill and meticulous observation. We need to observe and study the diet, lifestyle and environment of the patients having SLE and compare it with those who do not have it. But hardly anyone studies them properly these days. No useful conclusions are drawn, even my observations may be criticised due to lack of data from double blind studies, because people have forgotten the fact that it is impossible to have data from double blind studies for aetiology of diseases. We keep on adding junk evidences to the already evidence burdened Medicine; it is a herculean task now to choose from the evidences already available.
The first study established that SLE manifests more often with haematological problems, and the second was to validate Kozhikode Criteria [1,3]. The second study was done four years after the first study. Both studies included diagnosed cases of SLE from the Departments of Internal Medicine, Rheumatology, Haematology and Nephrology of Government Medical College, Kozhikode. Both newly diagnosed and the previously diagnosed cases under follow up were included. All the study subjects were followed up for a period of one year in both studies. There was overlap in the study subjects between both studies, due to the fact that patients already under follow up were also included. The first study included 108 patients and the second study included 71 patients; Male to female ratio was 1:10 and 1: 9 respectively in the studies.
Clinical diagnosis of SLE was considered in those presenting with undiagnosed clinical problems that were autoimmune in nature, like chronic ITP, hypothyroidism, autoimmune haemolytic anaemia, vitiligo, alopecia, or any other autoimmune disorder with some laboratory evidences to suggest autoimmunity. Complete haemogram including red cell indices, ESR, renal and liver function tests, urine routine, peripheral smear, ANA, anti-ds DNA were done for all cases. Only in relevant cases other investigations like reticulocyte count, Coombs test, complete ANA profile, radiological tests, tissue biopsy or cytology, bone marrow, lymph node biopsy were done. The Kozhikode criteria was used to diagnose those patients who did not satisfy the ACR criteria.
The most frequent clinical manifestations were Haematological. Large proportion of cases presented with haematological manifestation as the first symptom (Figure 15).
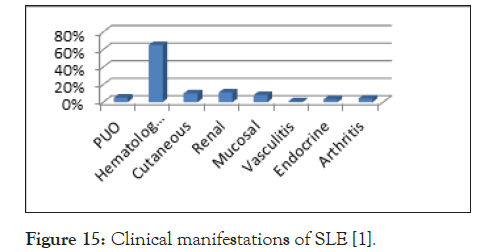
Figure 15. Clinical manifestations of SLE [1].
The most frequent clinical manifestations were Haematological. Large proportion of cases presented with haematological manifestation as the first symptom (Figure 15). Between musculoskeletal and haematological manifestations there was an inverse relationship (p value<0.001). There were 10 cases of hypothyroidism and 6 of autoimmune hepatitis, such presentations also are not given any representation in the ACR criteria [1]. The most common haematological manifestation was Immune Thrombocytopenic purpura (Figure 16).
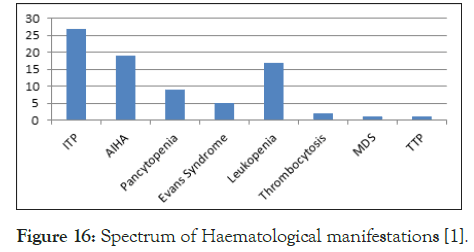
Figure 16. Spectrum of Haematological manifestations [1].
Anaemia was present in 62.9% cases and it was due to nutritional, autoimmune and anaemia of chronic disease. The commonest manifestation in those who had APLA was Cerebral Venous Thrombosis (CVT) and abortions. Thrombocytopenia was seen in 7 cases of APLA (Figure 17).
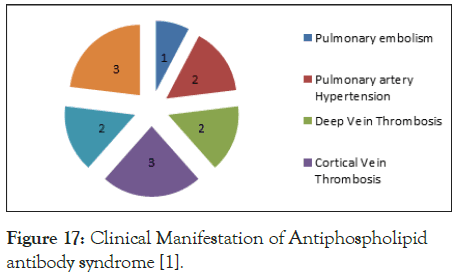
Figure 17. Clinical Manifestation of Antiphospholipid antibody syndrome [1].
It was observed 16 patients who were diagnosed as SLE with the Kozhikode criteria satisfied the ACR criteria only after long periods of follow ups as indicated in (Table 1).
| Serial number | First presentation diagnosis | Time from diagnosis to satisfying the ACR criteria(in months) |
|---|---|---|
| 1 | ITP | 72 |
| 2 | ITP | 32 |
| 3 | ITP, Hypothyroidism | 40 |
| 4 | AIHA | 7 |
| 5 | AIHA, Nephrotic Syndrome | 64 |
| 6 | Polyarthralgia alone | 4 |
| 7 | APLA, Very high ESR | 14 |
| 8 | CVA, ITP, Very high ESR | 144 |
| 9 | Anemia, Splenomegaly | 6 |
| 10 | Secondary Sjogren’s syndrome | 4 |
| 11 | Thyrotoxicosis | 11 |
| 12 | Polyarthralgia | 8 |
| 13 | Polyarthralgia | 4 |
| 14 | Anemia, Very high ESR | 12 |
| 15 | Pancytopenia | 12 |
| 16 | MDS | 60 |
Abbreviations: ACR: American College of Rheumatology; ITP: Immune Thrombocytopenic Purpura; AIHA: Autoimmune Haemolytic Anaemia; MDS: Myelodysplastic Syndrome; CVA- Cerebro vascular Accident.
Table 1: Time needed to satisfy the ACR criteria among 16 cases diagnosed with Kozhikode Criteria [1-3].
The second study, done 4 years after the first, was aimed at validating the Kozhikode Criteria. There were a total of 71 cases, out of this 30 were new cases and 41 were previously diagnosed cases, already under follow up. Both the ACR and the Kozhikode Criteria were applied to all diagnosed cases. All the cases diagnosed as SLE using the Kozhikode Criteria were also subjected to the ACR criteria. We found that large number of them did not satisfy the ACR criteria, but all those who satisfied the ACR criteria also satisfied the Kozhikode Criteria. Of the 71 subjects only 22 satisfied both ACR criteria but all of them satisfied the Kozhikode too.
But there were 45 subjects who satisfied only the Kozhikode Criteria, and 4 did not satisfy either criterion (Figure 18).
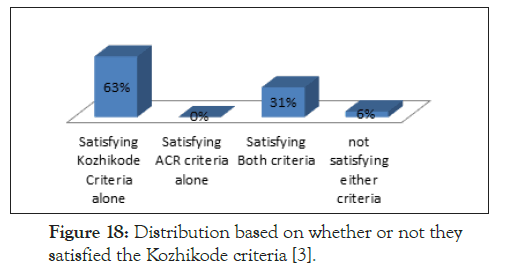
Figure 18. Distribution based on whether or not they satisfied the Kozhikode criteria [3].
Thus 67 subjects satisfied the Kozhikode Criteria at the beginning of the study but there was no patient who satisfied only the ACR criteria but not the Kozhikode criteria, which had validated the new criteria. Among the 30 new cases, 26 subjects satisfied the Kozhikode criteria, whereas only 6 were satisfying the ACR criteria. The 26 newly diagnosed cases, which satisfied the Kozhikode criteria alone, were followed up for a period of 6 months and it was observed that only 2 of them satisfied the AR criteria even at the end of 6 months. In addition, we have observed that those who were already under follow up after diagnosis using Kozhikode criteria, satisfied the ACR criteria only after long periods of follow up. Thus we could conclude that the Kozhikode Criteria helps in early diagnosis of SLE [3]
For studying the influence of diet and life style, we took matched controls. Dietary details were assessed using a food frequency questionnaire made for non-communicable disease survey, taken from the Integrated Disease Surveillance Project. Majority of the patients belonged to moderate socio economic status and had a sedentary life style (p<0.05) [3] (Figure 19).
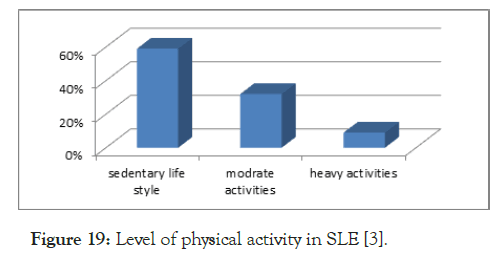
Figure 19. Level of physical activity in SLE [3].
Comparison of diet among cases and controls showed that there was a statistically significant decrease in intake of protein (pulses), vegetables and fruits (p=0.000). The intake of junk food and canned food was statistically significant among cases than in controls (p=0.000).
Major/Essential Criteria
1. Presence of an unresolved autoimmune disorder, which is known to occur with SLE (Chronic ITP, Immune hemolytic anemia, autoimmune hypothyroidism, autoimmune hepatitis/ cholangitis etc)
2. No other causes identified, other than autoimmunity, for the said problem, after good clinical reasoning and investigations.
Minor Criteria
1.Another co existing autoimmune disorder/any other clinical or laboratory evidence of autoimmunity
2.Positive ANA
3.Positive anti ds DNA
4.Sustained and definitive response to steroids and immunosuppressant even after 6 months of follow up.
Major ones are essential and any two or more of the minor criteria is diagnostic of SLE/Systemic autoimmune disorder (SAID)
ACR Criteria for Diagnosis of SLE
1.Malar rash
2.Discoid rash
3.Photosensitivity on exposure to UV light
4.Oral ulcers
5.Arthritis
6.Serositis
7.Renal disorder
8.Neurological disorder
9.Haematological disorder(Haemolytic anaemia, leukopenia, thrombocytopenia)
10.Immunologic disorder like Anti Ds DNA, anti-Sm and or antiphospholipid antibodies
11.Antinuclear antibodies.
If any of the four are present at any time during the course of the disease, a diagnosis of SLE can be made with 98% specificity and 97% sensitivity.
The list of haematological presentations in SLE is quiet long. These are chronic ITP, autoimmune haemolysis, autoimmune B12 deficiency, iron deficiency anaemia due to chronic low grade intravascular haemolysis, Evan’s syndrome (ITP+AIHA), pancytopenia (due to MDS, autoimmune Hypoplasia, Myelofibrosis). It could be refractory anaemia due to MDS, pure red cell aplasia (PRA), or B12 deficiency; sometimes presenting as leukopenia alone, antiphospholipid antibody syndrome alone with venous thrombosis or other vascular manifestations, lymphadenopathy with or without hepatosplenomegaly and fever-mimicking acute leukaemia, lymphoma, vascular purpura, vasculitis with thrombocytopenia, acquired clotting factor deficiencies, even occasionally as HELLP syndrome or TTP and apparent HUS like presentation. Any one of them or a combination of them could be the present in a given patient simultaneously or sequentially. But the point is that these manifestations could be seen only by those who deal with diagnostic problems. In these situations, the diagnosis of SLE might is often overlooked if one does not maintain a high index of suspicion and sticks to principles of clinical judgement, rather than depending too much on the laboratory data. In addition to the haematological problems we had patients who presented with other autoimmune manifestations like acute disseminated encephalomyelitis, cholangitis, hypersensitivity vasculitis, polyarteritis type, or Takayasu type of vasculitis, hypothyroidism etc and later on developing one or more of these haematological problems. We had at least twenty patients with SLE who presented as ITP alone. Sometimes they come with vasculitis and thrombocytopenia simultaneously. There were four patients with ITP and hyperthyroidism; another 4 with ITP with chronic active hepatitis, ITP and APLA were seen in 5 of them, MDS in two of them, Myelofibrosis in one patient. There were four patients with simultaneous manifestation of chronic hepatitis and DCT positive haemolysis. Two had autoimmune cholangitis with chronic liver disease; some had ulcerative colitis as the first presentation. One patient had autoimmune chronic active hepatitis and Myasthenia gravis both due to SLE. One of our patients was a 23 year old female who had ALL-L1, ten years ago, treated and cured but recently presented with high TLC, low Hb and thrombocytopenia which we thought could be relapse of acute leukaemia.
It is worthwhile remembering that the diagnoses in all these were primarily by good clinical evaluation, looking for evidence of an autoimmune phenomenon after ruling out other causes. Majority of them who initially had manifestations of autoimmunity in one tissue or organ alone were ANA negative and did not satisfy the ACR criteria, but did so on follow up [1-3]. Taking into account all the above factors, we realised the need for a practical guideline to diagnose SLE and framed a new criterion which we named as the Kozhikode criteria, and we have been using them for the last two decades.
SLE with ITP and later APLA syndrome
38 year old male driver in Quatar was diagnosed as chronic ITP fifteen years ago, investigations for SLE and infections were negative at that point of time. In 2004 he came to us when he developed a cerebrovascular accident with cerebellar infarct. Investigations then showed positive APLA(in very high titres) with positive ANA and Anti Ds DNA. He almost recovered with steroid, Azathioprine, Aspirin and anticoagulants though he had some residual cerebellar signs. Then he went back to Quatar, but after 5 years, he was noncompliant in his treatment, came back again when he had a sudden onset weakness of both lower limbs which on evaluation was sagittal sinus thrombosis. All medications, including aspirin and anticoagulants were restarted. But due to his poor compliance with treatment, he came back once again with features of multi organ involvement like digital gangrene, ARDS, hypotension, and CNS involvement. This time he was, febrile and tachypnoeic, but there was no pallor, jaundice, cyanosis, clubbing, or lymphadenopathy. There were haemorrhagic blebs on the tip of right thumb and palm, and tip of left middle finger (Figure 14).
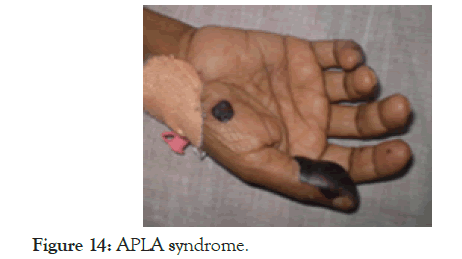
Figure 14. APLA syndrome.
Mild pedal oedema was present bilaterally. Pulse rate was 112/ min, all peripheral pulses were palpable, BP was 90/60 mm Hg. This time there was no evidence of deep vein thrombosis. He was drowsy, disoriented, pupils were equal and reacting to light, moving all limbs, deep tendon reflexes were brisk with bilateral extensor plantar response, there was no neck stiffness. Hb was 10 g/dL, TLC 6300/mm3, DLC-P81 L15 M4, ESR-120 mm, Platelet count was-1.19 lakh/mm3, MCV was-87 fl, Urine routine examination was unremarkable. Blood urea was 88 mg/dL, serum creatinine was 1.7 mg/dL, Serum Na+ was 129 mEq/L, K+ was 4 mEq/L, LFT was normal, RBS-176, FBS-172. Prothrombin Time and aPTT were elevated; FDP and D-dimer were very high. Repeat ANA, AntiDS DNA and APLA-IgG were positive in very high titres. The features were consistent with catastrophic APLA syndrome-having wide spread thrombotic disease with multiorgan failure. Lab features supported the diagnosis by elevated FDP, depressed fibrinogen level and elevated D-Dimer. Why he developed all this? Not only that he was non-compliant in treatment and he continued to use Hans which he was using for the last 16 years, To add to that he used to eat lot of fast foods with very little consumption of vegetables and fruits.
Presenting as vasculitis, pancytopenia and myelofibrosis
This 48 year old lady came for progressive pallor, fatigue, and a moderate sized splenic enlargement; the investigations showed pancytopenia; Hb was very low 4.2 g/dL, MCV 80 fl, RDW 13%, TLC was 2400/mm3, with a DLC of P60 L40 and the platelet count was 18,000/mm3, ESR was 80 mm. Peripheral smear did not suggest any diagnosis but bone marrow biopsy showed myelofibrosis. Since there was a small lymph node in the axilla and since she had healed vasculitis lesions on the lower limbs (Figure 13).
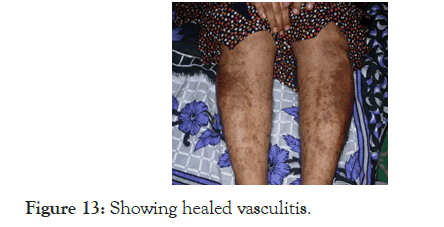
Figure 13. Showing healed vasculitis.
Lymphoma with vasculitis was the first possibility and an autoimmune aetiology was kept as the second possibility. But the lymph node biopsy was inconclusive, and the ANA and Anti Ds DNA were strongly positive. On starting Cyclophosphamide pulse and steroid the pancytopenia improved, splenomegaly almost disappeared and now she is on maintenance with Azathioprine.
53 years old male, came with fever of one month duration and generalized lymphadenopathy, hepatomegaly and just palpable spleen. There was pancytopenia and very high ESR, but the lymph node biopsy diagnosed as follicular lymphoma, with CD20+, BCL 2+, and the bone marrow trephine biopsy showed focal lymphoid cell/plasma cell infiltrates. Going by the principle of clinical medicine, lymphoma or any neoplasm should be diagnosed only after of excluding other disorders, and since the ESR was very high, and since there was plasma cell infiltrates also in the bone marrow, we asked for ANA and anti-ds DNA; both were positive [ANA 3.8 (<1), anti-ds DNA:49 (<20)]; he was doing well with Azathioprine and steroid, there was improvement in symptoms and signs and blood counts and the ESR became normal; after 9 months, someone doubted the diagnosis the treatment was stopped, then he came with erythematous skin lesion all over the body and face and palatal lesion which is very typical of SLE (Figures 11 and 12).
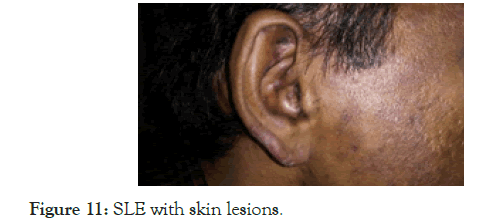
Figure 11. SLE with skin lesions.
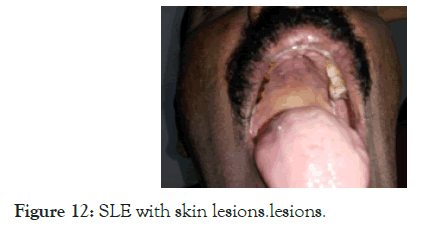
Figure 12. SLE with skin lesions.lesions.
The investigations this time was Hb: 9.6 gm/dL, MCV 92 fl, RDW 14.6%, platelet 83000/mm3, TLC 1370/mm3 (P78L15E3), ESR 106 mm, SGPT 59 IU, Alb/Globulin:3.5/3, ANA 5 (<1) anti- ds DNA 790(<20), Retro-negative, urine showed 2-4 pus cells, albumin-trace; This time confidently diagnosed as SLE relapse, restarted on Azathioprine, Hydroxychloroquine and steroid, he is on follow up for the last five years and is totally asymptomatic now.
Lymph node enlargement mimicking lymphoma
This 22 year old female came with non-cardiac chest pain as the only symptom. There was no fever, night sweats, weight loss or arthralgia. Except a 2 × 2 cm lymph node in right axilla, there were no other positive findings on examination and her hemogram was normal, but the ESR was very (120 mm). Since Mantoux test was negative, lymphoma was considered, but the lymph node excision biopsy report as Kikuchi’s disease. At this point ANA and Anti Ds DNA were asked for, both came as strongly positive. Lymph node enlargement in neck and axilla is seen in around 50% of patients with SLE during activity, but they usually are small.
Presentation mimicking acute leukaemia
This was a 21 year old female, who presented with prolonged fever of six weeks duration, was evaluated as a PUO from another centre. There was generalised lymph node enlargement, firm enlarged liver of 2 cm with just palpable spleen. Hb was 10 g/dL, TLC was 2400/mm3, with a DLC of P60L36M4, the platelet count was 130,000/mm3 and ESR was 130 mm. Acute leukaemia or other disease with infiltration of the bone marrow was considered, but peripheral blood film and trephine biopsy of the bone marrow examination were normal. At this point lymph node biopsy was done which was reported as necrotising lymphadenitis (Kikuchi’s disease). ANA and Anti Ds DNA were done at this point, which were positive with high titres. On steroid she became asymptomatic, pancytopenia and organomegaly disappeared and then she was continued on azathioprine followed by maintenance with Hydroxychloroquine.
Presenting as ITP first and then developing autoimmune haemolysis (Evans syndrome)
This 20 year old girl, fourth child of a non-consanguineous marriage, was diagnosed to have acute ITP in 1997 when she was 8 years old. Then she remained asymptomatic till 2003, this time came with purpura and ecchymotic patches all over the body, with a platelet count of 11000/mm3. She was then taking some homeo medicines for hypo pigmented patches on the face. There was no history of consanguinity or any family history of bleeding disorder. Chronic ITP was diagnosed in 2003; investigations for SLE were inconclusive, and were on follow up with. Two years after this in 2005, she developed menorrhagia due to low platelet counts and iron deficiency anaemia due to menorrhagia. This time physical examination showed vitiligo on the lips and investigations revealed showed positive ANA and borderline positive Anti Ds DNA. She remained asymptomatic on steroid and Azathioprine maintenance for 2 years, followed by Hydroxychloroquine for another 2 years. Due to a marriage proposal they stopped all the medicines and after six months there was severe thrombocytopenia, wet purpura, bleeding from GI tract and haematuria with DCT positive autoimmune haemolytic anaemia. At this point pulse methyl prednisolone and IV Ig were used but there was no response-finally she was given high dose dexamethasone, with which the platelet count improved. She was restarted on Azathioprine and is being continued now. Investigations this time showed higher titres of ANA and Anti Ds DNA. Her dietary habits, since child hood was abnormal as in the other patients.
SLE is the prototype of all autoimmune disorders; the problem of autoimmunity may be limited to one organ or sometimes affects several organs. Single organ autoimmune disorders include Hashimoto’s autoimmune thyroiditis, autoimmune haemolytic anaemia, ITP, Myasthenia gravis, pemphigus vulgaris, vitiligo and alopecia. They have a tendency to overlap and occur together in a given individual and they can run in families [1]. When they run in families it might suggest that it is due to genetic predisposition. But the case histories given below and our studies and observations suggest the possibility of them sharing the same diet, lifestyle and environment. It is also our observation that when two or more autoimmune disorders overlap in a given patient it could be SLE which is better called systemic autoimmune disorder (SAID) [2]. Some patients with single autoimmune disorder are already having full blown SLE or they are waiting to evolve into SLE. Only prolonged and proper follow up of patients will establish this link as well as the link to diet and lifestyle. Absence of a referral system with primary care doctors for proper clinical evaluation and good follow up and the doctor-shopping by patients prevent us from getting insight, or even loss of the already acquired insight, into aetiopathogenesis of diseases these days.
Statistics analysis
It should always be a diagnosis of exclusion and there should be the following features:
1.Presence of an undiagnosed clinical problem with on-going inflammation or dysfunction of any organ or organs
2.Presence of another autoimmune disorder
3.Presence of auto antibodies or evidence of cellular injury to self
4.Demonstration of a specific auto antibody or infiltration by lymphocytes in the pathologic lesion
5.Demonstration that the relevant auto antibody or T cells can cause tissue pathology.
Eg: By Trans placental transmission, or by adaptive transfer to animals, showing that there is an in vitro impact on cellular function. But in practice any two of the first four with supportive evidences is enough to make a diagnosis, if that is the case SLE also is no exception [3].
The supportive evidences to be made
1.Absence of any infection or any other cause for the problem being evaluated
2.Presence of another disorder which is autoimmune in nature
3.Sustained benefit with steroid and immune suppressants
Presenting as autoimmune haemolytic anaemia, autoimmune hepatitis and APLAS
This was as 23 year old female seen in 1996 and is still under follow up; she was referred to us for evaluation of the haemolytic anaemia, jaundice and raised liver enzymes in 1996. Clinically she had haemolytic anaemia but DCT was negative, though the cause of haemolysis was not obvious but was treated elsewhere with steroid. When she improved and became asymptomatic steroid was tapered and stopped. One year after this, she developed increasing jaundice, low grade fever with elevated liver enzymes. It was diagnosed as viral hepatitis and investigated, but all the serological markers for infective hepatitis were negative. When the haemoglobin started falling she was referred to us for a second opinion with a diagnosis of Wilson’s disease. On evaluation at our centre, this time DCT was positive. Thus SLE became a strong possibility being an young female, with autoimmune haemolysis and very high ESR, and there were no reasons to suspect Wilson’s disease. Infections were anyway excluded. Both ANA and Anti Ds DNA were positive and she was started on steroid and Azathioprine and was improving. While on treatment she one day noticed puffiness of left eye with blocked left nostril and conjunctival oedema. There was restriction of eye movements and the findings were diagnostic of cavernous sinus thrombosis. Mucor mycosis was suspected clinically and she was given Amphotericin injections for two weeks and made a prompt recovery (Figure 9).
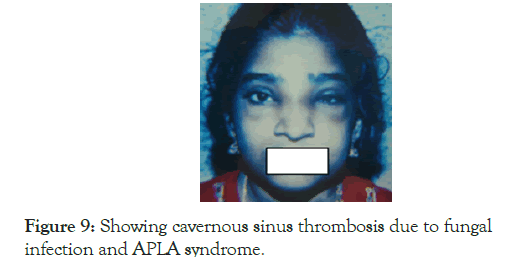
Figure 9. Showing cavernous sinus thrombosis due to fungal infection and APLA syndrome.
If we had waited to confirm the aetiology of fungal infection we would have lost the patient or her eye sight at least. Six months after this, while still on treatment, she became pregnant and her APLA screening was positive. Being the first pregnancy and since they gave a history of infertility of three years, pregnancy was allowed to continue and she continued to receive Azathioprine, Aspirin and heparin and reached full term. Unfortunately the baby had an intrauterine death due to umbilical cord around the neck. At the next pregnancy heparin and aspirin were added immediately after missing the period and continued till delivery of a normal healthy baby. Thus her final diagnosis was Autoimmune Haemolysis, Chronic Active Hepatitis Antiphospholipid syndrome, and infertility, all due to SLE. This patient is still under follow up in 2019, at the time of writing this.
Presenting as pulmonary hypertension, central cyanosis and autoimmune haemolysis
This was a 21 year old female, first came in 2004, and now has completed 15 years of follow up with us. At the initial presentation she was worked up in reputed centres when she presented to them with haemoptysis in 1999. They detected cyanosis, polycythaemia and severe pulmonary artery hypertension with no identifiable cause. Finally she was diagnosed as Primary Pulmonary Hypertension with R to left shunt across patent foramen ovale as in the previous case. All investigations for APLAS and SLE were non-contributory. But the clinical question that should come up in such situation is why it is primary pulmonary hypertension and why it is not something else, especially since it is 21 year old female? If we go by the principles of clinical judgment, taking into consideration of her age, gender and clinical presentation, it was not difficult to think of recurrent pulmonary thromboembolism resulting from APLAS or a vasculitis involving pulmonary arterial tree due to possible SLE (Figure 8).
Figure 8. Patient with APLAS due to SLE causing Primary pulmonary hypertension.
Luckily for us the repeat testing of ANA, anti-Ds DNA came positive with high titres of APLA. It is prudent to ask the question, if the antibodies are negative, should we keep aside the clinical conclusion? The answer should be a big NO. This patient was managed with venesection, steroid, anticoagulation followed by low dose aspirin and Azathioprine maintenance, and is still doing well and even the cyanosis disappeared. In 2009 she developed acholuric jaundice which on evaluation was DCT positive autoimmune haemolysis. Steroid was added again to control the haemolysis, and the dose of Azathioprine was increased. Tadalafil was added to control the severe pulmonary artery hypertension to which she seemed to respond well. What is highlighted by the above two case histories is that, once we make a strong clinical judgement, no test on earth can rule out that possibility. Laboratory tests are useful, only when the tests are consistent with the clinical diagnosis, they help us only to rule in and not to rule out a diagnosis. It is saddening to see the present trend of ignoring the clinical judgement once the laboratory tests are inconclusive.
Presenting as Pulmonary Hypertension, Cardiac failure with Cyanosis and polycythaemia
This was a 22 year old female who came in 2002; she was admitted in a very critical state with severe cyanotic heart disease, polycythaemia and congestive cardiac failure with very high JVP following a respiratory infection. Relatives brought her without any hope for recovery, since she was diagnosed to have Primary Pulmonary Hypertension with R to L shunt across patent foramen ovale, from a most reputed referral centre outside the state. The doctors and the relatives thought that nothing could be offered to her at this stage. The relatives of the patient were even ready to take her home for a peaceful terminal event (Figure 7).
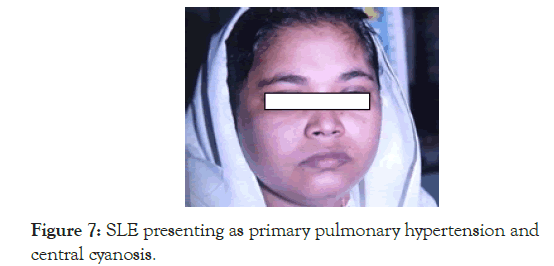
Figure 7. SLE presenting as primary pulmonary hypertension and central cyanosis.
We requested them to stay back to see what else can be done, applying the principle of clinical medicine, we should never accept a previous diagnosis without careful clinical analysis, even if it was worked up at a reputed centre. We reviewed the history and came to know that she had repeated abortions in the past with and used to get aches and pains all over the body. Antiphospholipid antibodies, ANA and Anti Ds DNA were negative, hence the possibility of SLE was ruled out at the previous centre. We arrived at a clinical judgement of recurrent pulmonary thromboembolism producing pulmonary hypertension due to APLAS and underlying SLE considering her age and gender too. Overall presentation was suggestive of evolving SLE, presenting apparently as primary APLAS. The haematocrit was 68%, with normal white cell and platelet counts, and normal liver function tests. We started intravenous dexamethasone, antibiotics, digoxin, diuretics, heparin and repeated venesections. There was remarkable improvement in all her symptoms and signs. Then she was maintained on oral anticoagulants, aspirin, diet and lifestyle changes and Azathioprine. When she improved we had repeated ANA, Anti Ds DNA and tests for antiphospholipid antibodies and to our surprise all of them were borderline positive. She was apparently asymptomatic and lived comfortably for another three years but with another episode of severe respiratory infection and more severe cardiac failure she succumbed to her illness. But the point to remember here is that if the previous centre had made a proper clinical diagnosis as APLA and SLE, rather than blindly depending on the lab reports alone, the patient could have been saved. The case history again underscores the importance clinical evaluation and clinical judgment.
Presenting with Hyperthyroidism and subsequently as ITP
This was a fifty five year old lady who came to us in 1997. She had unexplained loss of weight and fatigue with no diagnosis after evaluation. Since no other cause could be identified hyperthyroidism was considered as a clinical possibility. On further inquiry she has history of increased bowel movements which was diagnosed as Irritable Bowel Syndrome. On examination she appeared restless and the pulse volume was high. Subsequent evaluation confirmed the diagnosis of hyperthyroidism even though there was no eye signs, tremor or thyroid enlargement (Figure 6).

Figure 6. Hyperthyroidism with no external features to suggest.
While on Neomercazole for one year, she gained weight and her bowel symptoms disappeared; she was totally symptom free for two years. Four years after in 2001, she came with petechiae and purpura, and a diagnosis of ITP was made. Just because of the high index of suspicion SLE was considered in the differential diagnosis and also since she had past history of hyperthyroidism. ANA and Anti Ds DNA were asked for and both came as positive. She never had any joint or skin manifestations at any time during course of her illness.
She was put on steroid followed by Azathioprine for two years and presently she is on maintenance with Hydroxy chloroquine. Again the question is what will we do if the screening tests for SLE are negative or borderline in such a situation? Our conclusion is that we still have to entertain the diagnosis of SLE because she had two autoimmune disorders and also being a female, though of older age.
Presenting as Deep vein thrombosis due to Antiphospholipid Antibody Syndrome (APLAS)
25 year old female with a past history of foetal loss presented with deep vein thrombosis of the left lower limb. After evaluation she was diagnosed to have Antiphospholipid antibody syndrome, with positive Antiphospholipid antibodies, positive ANA and Anti Ds DNA, with no joint involvement so far. If the ANA and Anti DsDNA were negative we might call it as a primary APLA syndrome. What we call as primary is often not primary or idiopathic the secondary cause is not obvious. In case the patient continues with the present diet, lifestyle and environment, sooner or later she will come with all the features to satisfy the criteria to diagnose SLE. One should make a diagnosis of primary APLA syndrome in a young female like this with recurrent foetal loss and deep vein thrombosis even if the tests for SLE are initially negative.
Presenting as MDS
This 24 year old lady came with refractory anaemia and pancytopenia and the bone marrow was normal but hypercellular. There was no organomegaly and there was no other causes for the pancytopenia. The pancytopenia did not improve with folic acid, B12 injections and iron supplementation and a diagnosis of refractory anaemia possibly Myelodysplastic syndrome was made at another centre. When she was referred to us, we could elicit a history of recurrent unilateral painful parotid enlargement involving both sides, but never simultaneously, thus we kept the diagnosis of Sjogren’s syndrome. While under evaluation she developed another bout of parotitis (Figure 5).
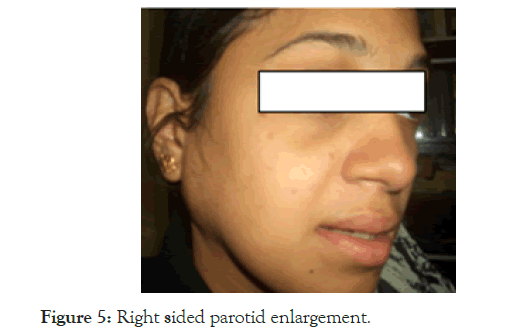
Figure 5. Right sided parotid enlargement.
Since her ESR was very high, SLE was considered, only because we were hesitant to accept the diagnosis of MDS; luckily both ANA and anti-Ds DNA came positive. After starting steroid and Azathioprine she became asymptomatic and is on regular follow up for the last fifteen years, till today she never had any skin, renal or joint involvement at any time during the follow up. The high ESR and the high index of suspicion only were helpful to diagnose her case. All autoimmune disorders show a tendency to overlap and turn into SLE at some time during the course.
SLE Presenting with severe Thrombocytopenia alone: This 25 year old female presented with wet purpura and severe thrombocytopenia (platelet 5000/mm3 ) and was diagnosed as ITP (Figure 4).
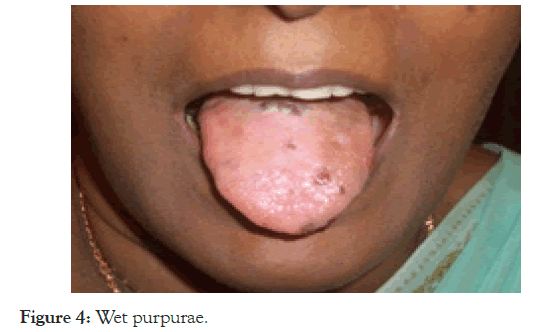
Figure 4. Wet purpurae.
On evaluation it was confirmed to be SLE with positive ANA and Anti-Ds DNA in the first presentation itself. The question that comes up here is, if the tests are not contributory, can we confidently rule out SLE? All chronic ITP in young females are possibly evolving SLE. Naturally the next question that arises is, how much are we justified in doing splenectomy for refractory ITP? Our observation is that we should avoid splenectomy at any cost. In the last three decades we had never opted for splenectomy in ITP and so far there was no mortality due to ITP.
Presenting as Iron Deficiency Anaemia, Autoimmune Haemolytic Anaemia, B12 deficiency, and Hashimoto’s thyroiditis. This 35 year old staff nurse was first seen in 2005, she had long history of fatigue and progressive pallor which was diagnosed as refractory iron deficiency anaemia with pica. She used to have the habit of eating mud and raw rice in spite of taking a diet which was reasonably good. She had these symptoms for several years and in 1997 she had an acute abdominal pain and severe melena and giddiness and received 9 units of blood transfusion. On upper GI endoscopy a gastric polyp of size 6 × 3 × 3 cm was detected and biopsy confirmed it as an ulcerated carcinoid tumour in the stomach. She underwent partial gastrectomy and was apparently asymptomatic for five years and then she had insidious onset of fatigue and progressive pallor and pica again in 2005. At this point on evaluation there was pallor, mild lemon yellow jaundice and the spleen tip was palpable. In addition there were external features of iron deficiency in the form of flat nails and of B12 deficiency with hyper pigmentation of knuckles and palmar crease (Figure 3).
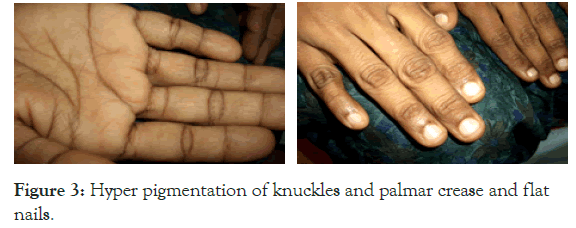
Figure 3. Hyper pigmentation of knuckles and palmar crease and flat nails.
There was diffuse enlargement of thyroid and the patient was clinically hypothyroid. Hb was 6.5, TLC, DLC was normal, Platelet count was 100,000/mm3, ESR 125, MCV 116 fl, Retic count of 12%, DCT was positive, and serum ferritin was very low, FNAC thyroid showed Hashimoto’s Thyroiditis, with high titres of Anti TPO antibody. All these work up was done in another tertiary care centre disorders but she was not improving on thyroxin, Iron and B12 injections given from that centre. It was obvious that she did not receive adequate doses of steroid and no other immunosuppressants were added due to lack of a definite diagnosis. She never had any clinical or laboratory evidence of renal, skin, joint or liver involvement.
On reviewing the history, the investigations and the very high ESR, we asked for ANA and Anti Ds DNA and both were positive in high titres. On adding adequate doses of steroid and Azathioprine and supportive treatment with iron, parenteral B12 and thyroxine supplementation she became asymptomatic. There were no joint symptoms at all in this patient too. More interesting point was that her initial symptoms of iron deficiency were due to chronic blood loss from the carcinoid tumour and later on due to low grade intravascular haemolysis as in the second case history. There is a possibility that the carcinoid and autoimmunity/SLE are related in some manner which is worth exploring in future.
Presenting as B12 deficiency and refractory iron deficiency anaemia due to autoimmune haemolysis: This patient was 36 year old when she first came in 1994, came with exertional syncope, dyspeptic symptoms, numbness and tingling sensation of both lower limbs of 3 months duration. She was diagnosed to have Iron deficiency anaemia which was not improving on haematinics and was referred for evaluation. The dietary history did not suggest any reason for iron deficneyv and there was no gastrointestinal blood loss, cola coloured urine or jaundice and no history of excessive bleeding during periods. She did not have any constitutional symptoms, arthritis or photosensitivity. On clinical evaluation she had severe pallor, koilonychia but no jaundice, lymphadenopathy of hepatosplenomegaly. Neurological examination showed all features of subacute combined degeneration as well. The diagnosis made was combined Iron and vitamin B12 deficiency, and the lack of response to haematinics at the previous centre was thought to be due to coexisting B12 deficiency.
Her Hb was 4.7 g/dL; TLC 5500/mm3 , with a differential counts of P62L35E3, and ESR of 165 mm in the first hour. Platelet count was 280,000/mm3. MCV was 104 fl. MCH was high and MCHC was normal. Blood urea, serum creatinine and bilirubin were normal. Peripheral smear and bone marrow suggested megaloblastic anaemia. Serum ferritin was low. Stool occult blood was negative. She was started on B12 injection, oral Iron, folic acid and Ranitidine, but there was no response. Desperately she was given one unit of packed red cell transfusion following which there was cola coloured urine suggesting intravascular haemolysis. At this point only the diagnosis was reviewed, because there was nothing to suspect SLE or an autoimmune disorder till then. Collagen vascular disease was considered because of the very high ESR, female gender, and evidence of haemolysis. Reticulocyte count was 8%, DCT was positive, ANA and Anti Ds DNA were also positive. She was then started on steroid and Azathioprine and continued the haematinics. While on treatment all symptoms and signs disappeared and was maintained on Azathioprine. After two years the patient was lost to follow up for a period of six months or so then she came back with reappearance of autoimmune haemolysis and typical skin lesions of SLE (Figure 2).
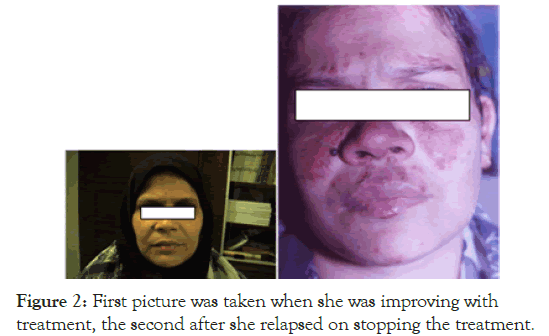
Figure 2. First picture was taken when she was improving with treatment, the second after she relapsed on stopping the treatment.
During the period she was on treatment from an alternative system called naturopathy. This again confirms that treatment for SLE is better with the modern medical approach. The refractory iron deficiency anaemia in her case was due to low grade intravascular haemolysis and haemoglobinuria, and most likely the haemolysis was complement mediated.
The refractory B12 deficiency, which did not respond even to parenteral B12, must be due to antibodies against intrinsic factor or parietal cells or even against the receptors for B12. But it was not worthwhile going after the mechanisms for each of the numerous manifestations so accurately with supportive evidences. Anyway in a resource poor setting it is not possible to investigate such things which will only add to the torture and agony of the patient. This time again she was started on Steroid and Azathioprine and maintained on the latter for more than 15 years. Recently she was not sticking to the lifestyle changes advocated and was depressed on the thought that she has an incurable disease and slowly started becoming non-compliant and came with nephrotic range proteinuria and refractory hypertension with all the features of Lupus Nephritis. Without any renal biopsy she was started on high dose dexamethasone and Mycophenolate Mofetil, then switched over to oral steroid and now in remission again at the time of writing this. This patient is on follow up for the last 25 years and at no time she had arthritis
Presenting as chronic ITP and diagnosed as SLE after 13 years, now completed 26 years follow up: This thirty year old female was first seen in 1993 for severe mucosal bleed and extensive purpura with very low platelet count and was diagnosed as Chronic ITP. Investigations for secondary causes including retroviral screening, ANA and Anti ds DNA were negative. She was given all combinations of treatment for ITP and since there was no response was kept on maintenance steroid but finally developed steroid induced Cushing’s syndrome and avascular necrosis of the head of femur bilaterally. At this point the patient went for indigenous treatment from a qualified Ayurvedic doctor, which helped to improve the platelet count and the cushingoid features disappeared with improvement in avascular necrosis and the treatment was continued for two more years. Then she discontinued that treatment because of the bitter taste of the medicine, after an year in 2006, that is thirteen years after the first diagnosis of ITP, she was brought with a platelet count of 5000 and severe bleeding. This time ANA and Anti Ds DNA were positive. After giving high dose steroid to bring up the platelet count, she was started on Azathioprine and low dose steroid again and maintained on Azathioprine alone. Even after 26 years of follow up in 2019, she does not have any arthritis or skin manifestations (Figure 1).
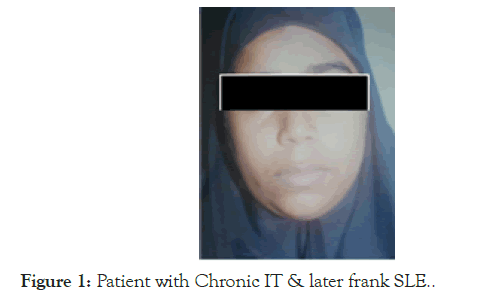
Figure 1. Patient with Chronic IT & later frank SLE.
She is now compliant with medications and is doing well, this story illustrates the fact that early initiation of Azathioprine was not done due to the negative results and pushed the patient into side effect of steroid and to indigenous treatment.
Systemic Lupus Erythematosus can present as an autoimmune disorder affecting a single organ or as a multisystem autoimmune disorder. Thus often it has a multitude of clinical features, making the patient visit any specialist, if doctor-shopping is encouraged. There would be presence of numerous auto-antibodies, circulating immune complexes and wide spread immunologically mediated tissue damage [4]. Ninety percent of cases are seen in women of child-bearing age group, with manifestations involving a single organ or multiple organs at a time. The hyperresponsive humoral immune system produce an array of autoantibodies against any antigen in blood, blood vessels, kidneys, skin, endocrine tissues, serosae, joints and CNS. Besides the auto antibodies against any antigen of the body, and there could be exaggerated response and consequent increased production of antibodies to commonly encountered viral antigens. Most manifestations of SLE are due to deposition of immune-complexes or in situ formation of immune complexes.
The first case in India was reported in 1955 [5]. The disease severity and its expression are largely determined by lifestyle, diet, genetic, demographic, environmental and geographical factors. The manifestations, serological expressions of the disease and morbidity could thus vary depending on the ethnicity and geographic regions [6,7]. Lifestyle practices and diet of the of population in India are often different in comparison to the west. It was observed in studies that those patients with Vitamin D deficiency have severe disease and the low levels of vitamin D on severity of SLE exemplifies the importance of dietary factors in SLE [8]. Interestingly Vitamin D deficiency is very high among Indians. Lack of a balanced diet, high melanin concentration in the skin and absence of outdoor activities and thus avoidance of sunlight contribute to low levels of Vitamin D 3 [9]; The same are the causes for other diseases as well [10].
The guidelines for diagnosis are framed based on the data form the western countries. It is only natural to expect differences in the clinical presentations in other places and races due to the entirely different diet, life style and environmental factors. SLE is one disease where we can find antibodies directed against any antigen in the body and since blood and the vascular tree possess more varieties of antigen as compared to all the other organs put together, it is only logical to expect maximum manifestations as haematological [1,2]. It is not logical to see SLE as a rheumatology or dermatology problem since the manifestations of the disease are more often in the blood.
The guideline used for diagnosis of SLE till 2012 was the American College of Rheumatology (ACR) Criteria, which is not really diagnostic criteria, but can help only in classifying the disease as SLE when someone has experienced several clinical features sequentially or simultaneously, it does not help us in early diagnosis. Only when four out of eleven criteria are satisfied one can say it is SLE. Thus to satisfy the ACR criteria the suffering patient has to wait for several years with the resultant agony and delay in treatment. All this is because of the fact that haematologic manifestations are not represented adequately in the ACR criteria.
Our observations and studies spanning three decades made us aware that haematologic manifestations are the commonest presenting problems in SLE, at least in this part of the world, and the diagnosis is confirmed only because of the high index of suspicion. Several of our patients had negative results on investigations but on follow up only they come up and then diagnosed as SLE. Joint symptoms as a presenting manifestation is rather uncommon or even rare and therefore one should not wait for joint symptoms to manifest to suspect SLE.
We have observed that the manifestations are in the following order of decreasing frequency
Haematologica l>Skin>Renal>Endocr ine>Eye>joint involvement>CNS involvement.
These observations are based on more than 300 proven SLE, all of whom came as diagnostic problems, not as established cases. This review article includes case histories to highlight the key observations, which lead to the development of ‘Kozhikode Criteria’ and the two studies on SLE.
Citation: Sasidharan PK (2019) SLE and Blood: The Kozhikode Criteria for Diagnosis. Lupus: Open Access 4:145. DOI: 10.35248/2684-1630.19.4.145
Received: 31-Oct-2019 Accepted: 15-Nov-2019 Published: 21-Nov-2019
Copyright: ©2019 Sasidharan PK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.