Organic Chemistry: Current Research
Open Access
ISSN: 2161-0401
ISSN: 2161-0401
Research Article - (2021)Volume 10, Issue 9
Additives are compounds added to enable desired balance of properties to be achieved for any particular application. The use of such ingredients, commonly stated as additives, has played an essential role in the commercial development of base materials. They are used for a variety of purposes: to improve processing, modify performance or appearance, prevent or retard aging and to reduce cost. This review article covers the most active heterocycles that have shown considerable actions such as stabilizers, preservatives, surfactants, retardants, curatives, accelerators, etc.
The broadest practical definition of anadditive is any substance that becomes part of a product either directly or indirectly during some phase of processing, storage or packaging. Direct additives, which are discussed in this publication, are those that have intentionally been included for a functional purpose by the processor, whereas indirect additives are those migrating into products in very small quantities as a result of growing, processing or packaging. Their quantities are small, yet their impact is great. Without additives, we would be unfortunately lacking in the abundant and varied products that we enjoy today.
Polymers are susceptible to degradation by the action of heat and light, especially in the presence of oxygen which is due to the structures of polymers themselves or due to the presence of impurities such as traces of transition metal ions and various chromophores. Most polymers absorb radiation down to wavelengths of 290nm1. Oxygen attack begins at weak spots of polymer molecule and proceeds by ionic and/or free radical mechanism. Carbonyl groups adjacent to an unsaturated bond are susceptible to photo-degradation after rearrangement of the α , β- to a β , γ- unsaturated group.

The degradation of polymers may be inhibited by the addition of antioxidants. The ratio of these chemicals to polymers is small, their effect is decisive for the endurance and life of polymers. Two mechanisms are considered to be important : chain transfer involving mainly peroxy radicals [for example phenols and amines] and peroxide decomposition [for example phosphite ester]. The amine antioxidants used in rubber industryare 6- dodecyl-1,2-dihydro-2,2,4-trimethylquinoline and polymeric 1,2- dihydro-2,2,4-trimethylquinoline. Peroxide decomposers are of two types :
sulphur containing and phosphite esters2.
Nearly all materials under appropriate conditions are subjected to deterioration brought about by micro-organisms and to curb their activity, preservatives (more appropriately referred as BIOCIDES) have become an important industrial additive. Preservation has now become a wide term and is applicable to edible as well non edible items. A sucessful industrial biocide should have a broad spectrum of activity, must be stable enough to withstand typical processing conditions, compatible with other ingredients and finally efficacy should be such that desired level of action is achieved at economical concentration.
|
Compound |
Application |
|---|---|
| 3,5 – dimethyl tetrahydro – 1,3,5 – thiadiazine-2-thione |
Preservative for adhesives, emulsions. |
| 5-chloro-2-methyl-4-isothiazolin-3- one | Preservative for cosmetics,personal care products. |
| Hexahydro-1,3,5-tris(2-hydroxyethyl)-s-triazine | Preservative for soluble cutting fluids. |
| N-(Trichloromethylthio)phthalimide | Fungicide for plastic, paints, resins. |
| 10, 10’-oxybisphenoxarsine | Preservative for vinyl plastics. |
| 2,3,5,6-Tetrachloro-4-(methyl- sulfonyl)pyridine |
Preservative for latex paints. |
Table 1: The sulphur – nitrogen compounds are the largest class of heterocyclic biocides for example, thiadiazine, isothiazoline and benzimidazole3.
These compounds are widely used as preservatives for latex paints, pigment slurries and as paper mill slimicide. 2-mercaptobenzo thiazole exhibits effective fungicidal properties. Quaternary ammonium compounds donot penetrate the cell but disrupt the semi – permeable membrane of the microorganism4.
Vulcanization refers to the process crosslinking to reduce plasticity thereby increasing strength and hardness. Heterocyclic compounds play an important role in this area of technology. Dithioamines5 are efficient vulcanizing systems producing high proportions of crosslinks. Most commercially useful compounds in this category are 4,4-dithiomorpholine(A) and 4- morpholinyl-2-benzothiazoledisulfide(B).
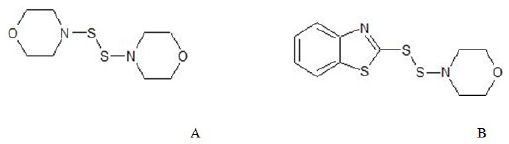
Accelerators are yet another organic compounds which increase the rate of vulcanization. Nitrogen compounds6 are very effective in catalyzing reactions leading to production of activated sulfur.For example, 2-mercaptobenzothiazole and dibenzothiazolyl disulfide.
This section deals with additives used to initiate and accelerate reactions of epoxides. Epoxy resins heve varied industrial uses. A number of imidazole derivatives [1] have been suggested as curatives for epoxy resins. These compounds provide long shelf lives at room temperature and cure at moderately elevated temperatures and are also less toxic [2-4].
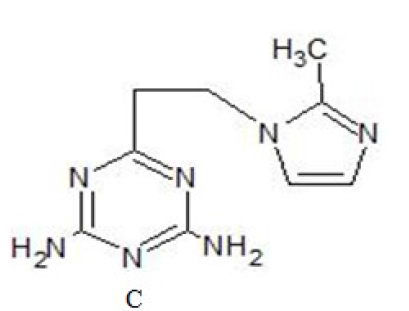
Both nitrogen atoms of imidazole are involved in the cure7 : the first step being reaction of the secondary atom with epoxy group and the second step the initiation of a catalytic polymerization by the tertiary nitrogen atom. More shelf life can be achieved by blocking the nitrogen atoms with various displaceable groups as is shown in C [5-8]. The resin viscosity drops initially upon the application of heat, passes through a region of maximum flow and begins to increase as the chemical reactions increase the average length and the degree of cross-linking between the constituent oligomer [9]. This process continues until a continuous 3-dimensional network of oligomer chains is created – this stage is termed gelatin. In terms of processability of the resin this marks an important watershed: before gelation the system is relatively mobile, after it the mobility is very limited, the microstructure of the resin and the composite material is fixed and severe diffusion limitations to further cure are created [10]. Thus in order to achieve vitrification in the resin, it is usually necessary to increase the process temperature after gelation. Cure monitoring methods give a significant insight to the chemical process and define process actions towards achieving specific quality indices of the cured resin systems.
Surfactants are widely used industrially and perform a variety of functions. As processing aids they help to disperse fillers and other ingredients and to form and stabilize emulsions. Anionic and non-anionic surfactants find many applications as wetting, spreading, emulsifying, dispersing and foaming agents. Cationic surfactants are mainly used as softners while amphoteric surfactants are less widely used.Cetyl pyridinium halides are used as germicides and sanitizing agents and morpholinium compounds find application in hair conditioning formulations.
Inhibitors are also surface modifiers. A metal surface can be regarded as a composite of localized electrodes connected through the bulk of the metal. In the presence of an electrolyte, for example surface moisture, these local-action cells are responsible for the chemical conversion of the metal to corrosion products.Organic corrosion inhibitors reduce or prevent these reactions; they are absorbed onto the metal surface and act by forming a barrier to oxygen and moisture, by complexing with metal ions or by removing corrodants from the surface. A number of nitrogen- and sulfur- containing heterocyclic compounds are effective corrosion inhibitors ,the imidazolines, benzotriazoles, quinaldic acid and 8- hydroxyquinoline being important types.
Oils, greases and waxes are used for the temporary inhibition of corrosion.They act as a barrier, preventing the condensation of water on the metal surface.Inhibitors such as piperidine8 and morpholine are incorporated to give added protection.
Some additional uses are summarized in this section.
The term blowing agent refers to those substances which act as the source of gas in the production of foamed materials.It is a chemical substance that is widely used in generating the gas to expand rubber, plastics and ceramics to create foam. In other words, it is called “baking powder” for rubber, plastics and ceramics.Compounds used as organic Chemical Blowing Agents are stable at ambient temperatures,but decompose at elevated temperatures to yield a large volume of gas, usually nitrogen. In general, the decomposition temperature should match the processing temperature of the polymer to be foamed.Tetrazole decomposes to give inert products which make it suitable for use with high temperature polymers.
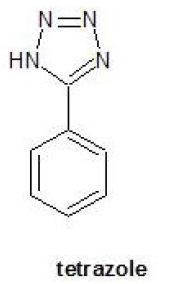
The decomposition tempereture becomes high when only blowing agent is used, and it may not satisfy the foam molding condition. In that case, the chemical added to adjust the decomposition temperature of blowing agent is called blowing agent activator.
The function of food preservative and antioxidants9 is to enhance the keeping ability, or stability of food products.Benzoic acid and its derivatives are among the most widely used antimicrobial agents; these, together with the propionates, sulfur dioxide, nitrates, nitrites and sorbic acid, account for the bulk of food preservatives. A number of those compounds find use, however, in various miscellaneous applications, including 3-acetyl-6-methyl-3H-pyran-2,4-dione as a mould and rope inhibitors in baked goods and hexamethylenetetramine as a preservative in some fish product.
Antimicrobials that destroy or delay the growth of bacteria, yeast and molds. E.g. nitrites and nitrates prevent botulism in meat products. Sulfur dioxide prevents further degradation in fruits, wine and beer. Benzoates and sorbates are anti-fungals used in jams, salads, cheese and pickles. Anti-oxidants that slow or stop the breakdown of fats and oils in food that happens in the presence of oxygen (Oxidation) leading to rancidity. Examples of anti-oxidants include BHT, BHA, TBHQ, and propyl gallate. Anti-enzymatic preservatives that block the enzymatic processes such as ripening occurring in foodstuffs even after harvest. E.g. Erythorbic acid and citric acid stop the action of enzyme phenolase that leads to a brown color on the exposed surface of cut fruits or potato.
Oxidation of the lipid structure in foods containing oils and fats produces carbonyl compounds whch are responsible for the flavour and odour associated with rancidity; the use of a suitable antioxidant can delay this process. Several heterocyclic compounds are among the antioxidants suitable for use in food; ascorbic acid and certain of its derivatives and erythorbic acid. The quinoline derivative is mainly used as an antioxidant in animal feed, but it can also be used to preserve the colour of paprika, chilli powder and ground chilli.
Flame retardants are compounds added to manufactured materials, such as plastics and textiles, and surface finishes and coatings that inhibit, suppress, or delay the production of flames to prevent the spread of fire. They may be mixed with the base material (additive flame retardants) or chemically bonded to it (reactive flame retardants. Mineral flame retardants are typically additive while organohalogen and organophosphorus compounds can be either reactive or additive.Most organic flame retardants contain at least one of the following elements : phosphorus, nitrogen, bromine10 or chlorine.
Citation: Mahima S (2021) Significance of synthetic heterocyclic gallows. Organic Chem Curr Res Vol. 10: 137.
Received: 14-Jan-2021 Accepted: 24-Aug-2021 Published: 06-Sep-2021 , DOI: 10.4172/2161-0401.21.10.484
Copyright: © 2021 Mahima S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.