
Biochemistry & Pharmacology: Open Access
Open Access
ISSN: 2167-0501

ISSN: 2167-0501
Original Research Article - (2019)Volume 8, Issue 1
Objective: The prognosis for chronic myeloid leukemia (CML) has been significantly improved in the era of tyrosine kinase inhibitors (TKIs) whose leader is Imatinib mesylate (IM). Obtaining a major molecular response (MMR) is a major objective to achieve. However, the difference in response between the two sexes is often controversial. This work aims to evaluate the molecular response according to sex in Algerian patients with chronic phase CML (CML-CP) treated with IM in first intention. Methods: All Novo CML patients benefited from molecular follow-up by quantification of transcripts by real-time quantitative PCR (RQ-PCR). The cumulative incidence of MMR (CIMMR) was estimated by the Kaplan-Meier method. The comparison was made using the parametric Log-Rank test. A value of P ≤ 0.05 is considered significant. Results: Fifty-five patients were included in this study, with a mean age of 45.7 ± 13.9 including 29 men (53%) and 26 women (47%). For women, the incidence of Mb3a2 transcript was higher than that of Mb2a2 (72% vs. 28%, P=0.12). Men, on the other hand, had an almost equal distribution (52% vs. 48%, P = 0.88). The CIMMR for men was lower than that for women (45% vs. 80%, P=0.018). Conclusion: Women seem to respond more favorably to IM than men.
CML; Sex; Transcript type BCR-ABL1; QR-PCR; MMR; Imatinib; Tyrosine kinase
In CML, the BCR-ABL1 gene is the result of a reciprocal translocation between the long arms of chromosomes 9 and 22: t (9- 22) (q34; q11), which fuses the gene coding for tyrosine kinase c -abl (Abelson virus oncogene cell homolog) located on chromosome 9 at breakpoint cluster region (BCR) located on chromosome 22, resulting in a shorter chromosome 22 called the Philadelphia chromosome (Ph1 Ch) [1]. This fusion gene encodes an abnormal chimeric protein with 210 kDa BCR-ABL1 (p210) that has a high deregulated tyrosine kinase activity (TKA) responsible for the leukemic process of CML [2]. The transcripts b3a2 (e14a2) or b2a2 (e13a2) (cut in Mbcr between exons e12-e16 also called b1-b5), are found in more than 95% of CML cases. The p210BCR-ABL1 chimeric protein has become the target of a therapeutic arsenal: the IM-mediated tyrosine kinase inhibitors (TKIs), which have transformed their prognosis by significantly improving the overall survival of patients reaching almost that of the general population [3].
The objective of our study was to determine if there was a difference between the two sexes with respect to the clinical-biological parameters and the status of the molecular response in patients with CML-CP treated in the first line by IM.
Patients: Patients with CML-CP treated by IM as a first line were included in this descriptive, observational, prospective study during 4 years from March 1, 2013 to April 31, 2017".
Methods
BCR-ABL1 transcript levels were measured by real-time quantitative QR-PCR in peripheral blood cell samples on a Rotor- Gene® analyzer using a standardized kit (ipsogen BCR-ABL1 Mbcr IS-kit), at the time of diagnosis, then every 3 months after the start of IM treatment according to the recommendations of the European Leukemia Net (ELN) [4]. Clinico-biological characteristics were collected at the time of diagnosis.
The results are expressed as BCR-ABL / ABL ratio (%) and adjusted internationally using a conversion factor of 0.152 included in the kit. According to their transcript level, patients are classified according to the ELN 2013 definition of response criteria (Table 1) [4].
| Optimal | Warning | Failure | |
|---|---|---|---|
| Baseline | NA | High risk Or CCA/Ph1, major route |
NA |
| 3 months | BCR-ABL1 ≤ 10% and/or Ph+ ≤ 35% | BCR-ABL1>10% and/or 35%<Ph+ ≤ 95% |
Non-CHR and/or Ph+>95% |
| 6 months | BCR-ABL1 ≤ 1% and/or Ph+=0 |
1%<BCR-ABL1 ≤ 10% and/or 0<Ph+ ≤ 35% | BCR-ABL1>10% and/or Ph+>35% |
| 12 months | BCR-ABL1 ≤ 0,1% | 0,1%<BCR-ABL1≤1% | BCR-ABL1>1% and/or Ph+>0 |
| Then, and at any time |
BCR-ABL1 ≤ 0,1% | CCA/Ph– (–7, or 7q–) | Loss of CHR Loss of CCyR Confirmed loss of MMR* Mutations CCA/Ph1 |
NA, not applicable; MMR, BCR-ABL1 ≤ 0.1% = MR3.0 or better; CCA/Ph1, clonal chromosome abnormalities in Ph1 cells; CCA/Ph–, clonal chromosome abnormalities in Ph– cells. *In 2 consecutive tests, of which one with a BCR-ABL1 transcripts level ≥ 1%. CHR, complete hematologic response; CCyR, complete cytogenetic response.
Table 1: Definition of the response to inhibitor of ABL1 in the first line of treatment (all the TKIs).
Fifty-five patients of mean age 45.7 ± 13.9 (19-78) were included in this study. 29 men (53%) and 26 women (47%) with a sex ratio M / W of 1.16. The median age at diagnosis was 48 years old. 33 patients (60.0%) had expressed b3a2, 21 patients (38%) hadb2a2 and only 1 patient (2%) had a rare e19a2 transcript. The epidemiological and clinical characteristics of our series of studies are summarized in Table 2.
| Sex | Type of Transcript (breakpoint) | Median sex | SOKAL N (%) | EUTOS | Hemogramme | |||||||
| b3a2 | b2a2 | e19a2 | F | I | E | F | E | WBC | PLT | Hb (g/dL) | ||
| (x109 g/L) | (x109 9/L) | |||||||||||
| Male | 15 | 14 | 0 | 45 | 7 | 13 | 9 | 22 | 7 | 270 | 436.62 | 10.19 |
| n=29 (53 %) | (52) | (48) | (24) | (45) | (31) | (76) | (24) | |||||
| Female | 18 | 7 | 1 | 47 | 7 | 11 | 8 | 23 | 3 | 190 | 535.92 | 10.84 |
| n=26 (47%) | (69) | (27) | (4) | (27) | (42) | (31) | (88) | (12) | ||||
| P value | 0.94 | 0.12 | 0.59 | 0.969 | 0.19 | 0.38 | 0.35 | 0.21 | ||||
Table 2: characteristics of patients based on combined sex.
Median follow-up was 24 months (6 to 48 months). At diagnosis the median BCR-ABL1/ABL1 ratio was 57% (14-98). The BCR-ABL1/ ABL1 ratio increased from a median of 57.00 IS at diagnosis to a median of 5.53 IS after only 3 months of follow-up (P <10-3), then at a median of 1 x 10-1 IS after 12 month of treatment (P <10-3) to reach median rates of 7 x 10-2 and 2 x 10-2 at 18 and 24 months (P <10-3) (Figure 1). The MMR rate was 52, 67 and 75% at 12, 18 and 24 months, respectively Figure 2.
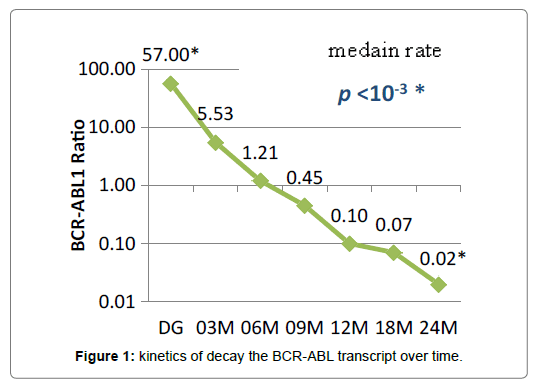
Figure 1: kinetics of decay the BCR-ABL transcript over time.
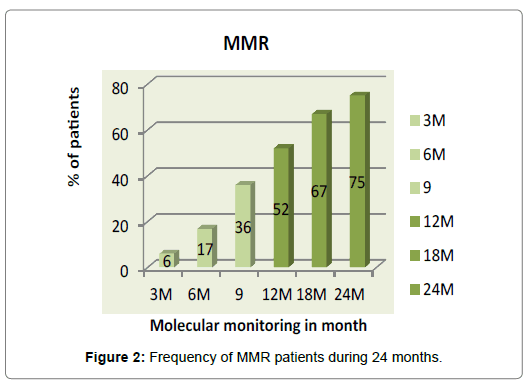
Figure 2: Frequency of MMR patients during 24 months.
The rate for men was lower than that for women at 18 months (58% vs. 70%, P=0.32) and 24 months (71 vs. 73%, P=0.61). The 18-month CML-CP was 80% (95% CI: 50-90%) for women and 45% (95% CI: 35.3-80%) for men (P=0.018) (Figure 3). The failure rate was estimated at 29% after 18 months of treatment. Of the 16 patients who failed initial treatment, 6 (37%) were female versus 10 (63%) male.
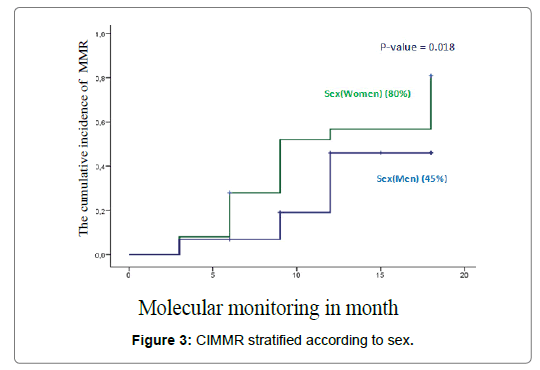
Figure 3: CIMMR stratified according to sex.
In this study, we compared clinical-biological parameters and therapeutic response with sex in patients with CML-CPand treated with IM as first-line treatment. Molecular tracking allowed us to stratify our patients according to the ELN treatment response definitions (Table 1) [4]. The judgment criterion taken into account was the obtaining of a MMR. This was defined at the end of the IRIS study by a reduction of more than 3log10 (Ratio ≤ 0.1%) of the expression level of BCRABL1compared to the theoretical value of 100% attributed to the diagnosis [5]. In our series, women were slightly older than men, but the difference was not significant (P=0.59).
The distribution of transcripts by sex does not find any significant difference. Our result is consistent with that found by Lin's team [6] In females, the b3a2 transcript (69%) was more common than b2a2 (27%) (P = 0.14), whereas in males the distribution of b3a2 and b2a2 transcripts was almost equal (52 vs. 48%) (P = 0.87). The incidence of b2a2 was higher in men compared to women (48% vs. 27%, P = 0.65) and b3a2 in women (69% vs. 52%, P = 0.52).
Our result is supported by the majority of studies, in particular that of the ELN cohort which constitutes the largest sample [7]. On the other hand, in a study involving 170 Indian patients with CML, Kagita et al, found a significant sex difference with a more frequent b3a2 in men (66.05 vs. 33.02%, P=0.061) [8]. No significant differences were found in prognostic scores, sokal and EUTOS. In particular, analysis of blood counts at diagnosis showed that men had a higher average number of leukocytes indicating a higher tumor burden than women (267 x 109 / L vs. 190 x 109 / L, P = 0.38).
Very significant reductions in the level expression of the BCRABL1 transcript over time (P<10-3) were observed. This rapid and considerable reduction in leukemic load is a direct reflection of the anti-tumoral effect of IM particularly in patients who respond well. The frequency of patients who achieved a reduction of at least 3 log (≤ 1 10-1) increased during follow-up with a median time to obtain the 12-month RMM [9-18].
One of the main findings of this study is that women had not only a significantly higher MMR-CP than men, but also a lower failure rate than men (37 vs. 63%, P = 0.26). Indeed, the sex-stratified MMR-CP curve (Figure 3) shows that at 18 months of treatment, men lag far behind women (45 vs. 80%) (P = 0.018). In addition, women tended to have an early optimal molecular response than men: 69% vs. 48%; (P = 0.097) at 3 months and 62% vs. 39% (P = 0.054) at 6 months. These data therefore suggest that the female sex is more favorable to a better molecular response than the male sex. Other recent studies have confirmed this superiority [6,9].
Branford et al. reported on a series of 423 patients newly treated with IM that female was one of the factors associated with MMR [9]. In a retrospective study involving 166 patients treated with IM for 10 years, Lin et al. [6], also found that men had a significantly lower MMR rate than women (63.3% vs. 81, 6%, P = 0.006) and also a shorter relapse time (median 354 vs. 675 days, P=0.049).
It appears that this difference may be related to lower adherence to men's treatment compared to women's, as well as poor treatment tolerance [10,11]. Pharmacokinetic variability could also have a major influence. Indeed, a correlation between the plasma concentration and the response to MI has been described. Indeed, women who were in molecular response had up to 30% higher concentrations of IM than men [12,13].
The difference in body weight could explain this difference in concentration. A lower weight than men means that women can receive an average dose of medicine in milligrams per kilogram higher than men, which may account for a higher plasma concentration [9]. The hypothesis of a transient immune response during the early stages of IM treatment is to be considered and could be a major factor in the dynamics and decrease in tumor burden of CML [14,15].
The type of transcript can also be incriminated. Indeed, several published studies have suggested that in patients expressing the b3a2 transcript, the response to treatment is faster and deeper [16-20]. In the recent study by Hanfstein et al, the authors found that the cumulative probability of achieving MMR and MR4.0 at 5 years was significantly higher in patients with type b3a2: CIMMR (85 vs. 81%; = 0.002) and CIMR 4 (76 vs. 58%, P <0.001), as well as for the median time to obtain MMR and MR4.0 which was also shorter in patients with type b3a2 with respectively (14.2 vs. 18.4 months) and (32.4 vs. 55, 2 months) [17]. The recent study by Castagnetti et al. also finds the same results. Patients expressing type b3a2 (52%) had a better probability of obtaining a MR3.0 at 18 months (67% vs. 52%, P = 0.001), and a MR4.0 at 36 months (30% vs. 20%, P = 0.013), as well as a median time to obtain significantly shorter compared to patients of type b2a2 (37%) (6 vs. 12 months for RM 3.0) and (41 vs. 61 months for MR 4.0) [20].
Lucas et al. showing a faster time to obtain b3a2 (P = 0.006), suggested that this difference was due to higher tyrosine kinase activity in patients expressing b2a2 compared to expressing b3a2 (P = 0.017), which means a higher TKA and therefore a higher resistance to treatment [16].
In our study, we found that the decay kinetics of the BCRABL1 transcript were faster in the b3a2 type than in the b2a2 type, as evidenced by the median rates obtained (0.095 vs. 0.29, P=0.52), (0.034 vs. 0.11, P=0.74) and (0.014 vs. 0.026, P=0.77), respectively at 12, 18 and 24 months of treatment. this allowed us to conclude that reductions of at least 3 log10 are obtained more rapidly in patients of type b3a2 than in those of type b2a2, and given that in our series women had a higher Mb3a2 transcript incidence than Mb2a2 (72% vs. 28%, P=0.12), this might partly explain their advantage over men in the therapeutic response, all the more so since the isotype b2a2, which was more frequent in men (14 vs. 7, P = 0.18), was associated with a higher average number of leucocytes indicating a higher tumor burden than in those with isotype b3a2 (321.54 x 109/L vs. 174.13 x 109/L, P=0.18).
The variability of the response could also be explained by the structural difference, particularly in the SRC domains (SH3, SH2, SH1) and the DNA binding domain of the p210 protein, thus reducing the activity of the kinase [21]. Indeed, the protein tyrosine kinase b3a2 (2031Aa) differs from b2a2 (2006 Aa) only by an insertion of 25 Aa (75 Pb). Junction sequences derived from BCR-ABL1 fusion can bind several class I and II molecules of the histocompatibility complex (MHC) and cause specific immune responses thus suggesting an additional immunological effect compared to the b2a2 protein [20,22].
Other factors may be involved including pharmacogenetics. Thus, genetic polymorphisms affecting drug-transporting genes could be one of the most important factors influencing the molecular response and leading to resistance to IM. One of the best known mechanisms is the overexpression of the protein responsible for the expulsion of IM outside the cell (efflux pump), p-glycoprotein (P-gp), encoded by the MDR1 gene (multi drug resistance), the consequence of which would be the decrease of the intracellular accumulation of the ITKs and the increase of their transport towards the extracellular medium thus causing a modification of the response to the IM (123, 210). SNPs may occur in the coding region of the MDR1 gene resulting in overexpression of P-gp. These SNPs concern exon 12 1236 T> C, exon 21 2677 G> T / A, and exon 26 3435 C> T [21].
Our study was able to confirm the literature data by finding that women had higher and faster molecular response rates to IM compared to men. This observation requires confirmation of a larger series by a broader prospective study. In order to individualize the dosages, and to optimize the therapeutic effect of circulating ITK, it would be interesting to do a therapeutic drug monitoring (TDM) of TKI.
Citation: Nachi M, Kihel I, Guella D, Dali-Ali A, Abed A, Boukhatmi Y, et al. (2019) Sex and Major Molecular Response to Imatinib Treatment for Patients with Chronic Myeloid Leukemia. Biochem Pharmacol (Los Angel) 8:263. doi: 10.35248/2167-0501.19.8.263
Received: 08-Jan-2019 Accepted: 01-Feb-2019 Published: 08-Feb-2019 , DOI: 10.35248/2167-0501.19.8.263
Copyright: © 2019 Nachi M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.