Emergency Medicine: Open Access
Open Access
ISSN: 2165-7548
ISSN: 2165-7548
Case Report - (2016) Volume 6, Issue 3
Rhabdomyolysis is a widely recognized yet rare complication in Statin use. Rhabdomyolysis might be triggered by prescription of high doses of Statins or by Statin accumulation due to interactions with concomitant medication. Muscle cell destruction as evidenced by myoglobin elevation can induce potentially life threatening acute renal failure known as crush kidney. Here, we report a case of a sudden severe rhabdomyolysis with consecutive renal failure in a patient who received low dose Statin- therapy for 6 years without previous complications.
Keywords: Rhabdomyolysis, Statin therapy, Statin- associated myopathies (SAM)
Statins are a common group of cholesterol lowering pharmaceuticals, with the shared pharmacodynamical characteristic of 3-HMG-CoA- inhibition, the key enzyme in cholesterol synthesis [1,2]. Inhibition of 3-HMG CoA by Statins is the most effective way of lowering low density lipoprotein cholesterol (LDL). LDL- levels correlate directly with cardiovascular mortality. Numerous randomized controlled trials confirmed and stressed the Statinmediated therapeutic benefit [3-5]. Statin significantly reduces the risk for cardiovascular death, for myocardial infarction, for stroke and the risk for arterial revascularization therapy [3-5]. Statin therapy aiming at lowering the cardiovascular risk is an established and well tolerated therapy for most patients with more than 3.2 billion prescriptions (313,1 mil DDD) and annual costs of 193.8 mil Euro in Germany in 2012 [6]. Adverse effects are rare. Severe complications occur in less than one in 10.000 patients [7]. To date, the definition of a Statin associated myopathy (SAM) remains unclear; especially the diagnostic criteria of a SAM are ill defined, which may explain different data concerning the prevalence and seriousness of SAM [8]. However, there is consent that Statin can cause several muscle related complaints. These may range from mild myalgia, may lead to manifest myopathy and myositis- mimicking symptoms and may culminate in severe rhabdomyolysis. The latter may - when not recognized early enough - induce crush kidney with consecutive renal failure and death [9,10].
Rhabdomyolysis as an adverse event was reported for all available Statins. The highest risk of Statin- induced rhabdomyolysis was associated with Cerivastatin which was taken off the market in 2001. This occurred 3 years after first approval of Cerivastatin which was responsible for more than 100 rhabdomyolysis- related deaths [11].
More recently, a self- limiting Statin- induced primarily toxic necrotizing myopathy can be distinguished from a persisting autoimmune mediated Statin- induced necrotizing myopathy (IMNM). The latter persists even after cessation of Statin therapy and immunosuppressive therapy [12]. In addition, Anti-HMG- CoAReductase- Antibodies can be detected in the serum and muscle of affected patients [8,13].
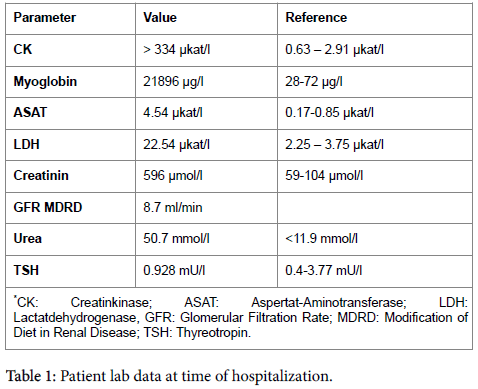
A 70 year old Caucasian male presented with a one year history of progressive symmetric generalized muscle weakness and myalgia. His past medical history was significant of chronic renal dysfunction (KDOQI IV), chronic heart failure NYHA III, coronary disease with acute myocardial infarction in 1988 and coronary artery bypass grafting in 2009, chronic atrial fibrillation under oral anticoagulation, hypertensive cardiomyopathy, diabetes Type II, hyperlipoproteinemia and obstructive sleep apnea syndrome (OSAS). He had a six year history of low dose Statin therapy of 40 mg/d. Further medication included ASS 100 mg/d, Rivaroxaban 15 mg/d, Metoprolol 95 mg/d, Ramipril 5 mg/d, Furosemid 40 mg/d, Molsidomin 8 mg/d, Isosorbiddinitrate 80 mg/d, Pantoprazol 20 mg/d and Insuline. Clinical examination on admission revealed proximal muscle weakness of all extremities (level of strength 3/5). Electroneurographic and myographic diagnostics showed chronic myopathic alterations in all of the examined muscles of upper and lower extremities. Alterations were more pronounced in proximal muscle groups of the lower extremities. Thyroid function was normal. Antibody diagnostics provided no typical findings for classical antibody positive autoimmune myositis (dermatomyositis, SLE, scleroderma-myositis-overlap-syndromes, Sjantibody positive autoimmune myositis). Creatinkinase (CK) was elevated with activities > 334 μkat/l. The patient developed a crush kidney with acute renal failure (Table 1) necessitating a continuous veno-venous hemofiltration (CVVH). Muscle biopsy was performed to confirm or exclude clinically suspected polymyositis. The clinical differential diagnosis was a chronic myopathy of unknown cause. An open biopsy was performed for the M. vastus lateralis of M. quadriceps femoris. Histology revealed numerous scattered muscle fibre necroses. Some necrotic fibres were in the state of macrophage mediated degradation (myophagocytoses). Despite wide spread, scattered muscle fibre necroses, the tissue was devoid of an interstitial or endomysial inflammatory infiltrate.
Immunohistochemistry highlighted a striking sarkolemmal und cytoplasmatic up- regulation of MHC class I- expression. Also, c5b9 (Membrane-Attack Complex) – positive complement deposits were detected on endothelial cells of endomysial capillaries and on the sarcolem and in the cytoplasm of necrotic muscle fibers (Figure 1). The histopathological diagnosis of SAM was made, followed by serum testing for the Anti-HMG-CR antibody. No Anti-HMG-CR antibody was detected. Simvastatin was withdrawn and the patient received Ezetrol as well as fluid resuscitation and high dose immunosuppressive therapy with prednisolone 100 mg/d. After 3 days of CVVH renal function was completed restored. And by day 15 myoglobin- and CKlevels had normalized (Figure 2). The patient was discharged without further complaints on day 18 after hospitalization.
Figure 1: A: Skeletal muscle with numerous, scattered necrotic fibres (*) without sings of inflammation (H&E × 100), B: Single skeletal muscle fibre in the state of myophagocytosis (H&E × 200); (*) marks another fresh single fibre necrosis, C: Detection of c5b9- positive immuncomplexes on small vessels (arrowheads), endomysial capillaries and sarkolemmal and cytoplasmativ in necrotic muscle fibres (c5b9 × 200), D: Clear sarcolemmal and zytoplasmatic upregulation of MHC class I (MHC-I × 100).
In clinical practice up to 10% of patients receiving Statin therapy develop at least mild forms of myopathy, an underestimated side effect supported by the Primo Trial [14]. Given the context of more than 3.2 billion prescriptions in Germany in 2012 a large number of affected or symptomatic patients are to be expected. Almost 30% of Statin associated incidences occur within the first year of treatment [15]. However onsets of muscular side effects have been documented between 2 month and up 10 years after initiation of Statin therapy [16]. Thus, a long standing uneventful and well tolerated Statin therapy as in our patient with sudden rhabdomyolysis after 6 years confirms these observations. Statin associated muscle problems vary considerably. Some patients with high CK level do not present with any muscle weakness until they reach a critical value [16]. On the other hand there are patients with distinct muscle parses at a lower mild CK level. Cessation of Statin medication usually leads to a fast recovery of muscle related symptoms.
The exact mechanism of Statin associated myopathies (SAM) still remains elusive. Several theories exist ranging from membrane destabilization due to decreased cholesterol content of skeletal muscle plasma membrane, impaired mitochondrial function due to coenzyme Q10 depletion, disturbed calcium metabolism and vitamin D deficiency [10]. Also, there are various co-medications that increase the risk of Statin associated myopathy, mostly by interference of the metabolizing cytochrome p 450 system (CYP3A4, CYP2C). Especially the fibric acid derivative gemfibrozil is known to aggravate symptoms and severity of Statin associated myopathy. The development of Statin associated muscle problems is dose-dependent [8]. Nevertheless severe cases of SAM have been reported in patients under low dose Statin medication and without interfering medication. Documented comedication in the presented case was devoid of potentially interfering drugs. However, personal communication with the patient`s family doctor revealed an additional Colchicine medication in close temporal vicinity of clinically manifest rhabdomyolysis. Colchicine was prescribed as muscle pain was misinterpreted as a potential goat attack. Colchicine itself can cause myopathy. Concomitant treatment of Colchicine and Simvastatin may exacerbate its myotoxic effect [17]. Before excretion Colchicine is metabolized in the liver by demethylation. Statin metabolism may compete with Colchicine for the CYP3A4-isoenzym leading to higher serum concentrations of both medications, thereby increasing the risk of side effects.
Of note, also genetic predisposition is discussed to cause Statin associated myopathies (SAM). Developing muscle symptoms are more frequent in patients with inborn metabolic muscle diseases such as McArdle disease or MADA-deficiency [8]. In these cases, Statin medication can unmask these underlying genetic muscle diseases [12]. Also mutations or polymorphisms of genes related to regulate serum Statin levels are known to cause a higher risk of developing SAM. Also genes responsible for muscle vascularisation, those affecting intracellular Statin concentration in muscle cells and genes responsible for muscle tissue energy metabolism are associated with SAM [15]. Metabolic disease (McArdle, MADA-deficiency) was excluded in the muscle biopsy of the presented case. Genetic testing was not performed. Advanced age, female sex, low body mass index, alcohol consumption are further predisposing factors for SAM. Given the high number of prescriptions clinicians are in need for reliable risk evaluation methods for the identification of vulnerable patients.
Clinical relevant rhabdomyolysis is a rare in Statin therapy. Mortality is low with 0.15 deaths per 1 million [18]. However, the FDA - Adverse Event Reporting System identified 147.789 case reports with suspected Statin associated myopathy between 2005 and 2011. The highest rate of rhabdomyolysis was observed for simvastatin [19]. Rhabdomyolysis is defined by CK levels at least 10 times normal and reflects acute and massive muscle fiber necroses and accompanied by the release of muscle related metabolites into the bloodstream [16].
Clinical monitoring of Statin associated myopathy may include baseline CK levels of patients especially if there are risk factors as impaired renal function, genetic myopathy in the past medical history or significant alcohol abuse [8,15]. Co-medication should be checked for potential interaction with Statins, especially if new medication is prescribed including herbal cures. Patients with muscle related symptoms and Statin medication should immediately checked for CK increase. Medication should be stopped immediately and CK levels monitored. In case of persistent elevated CK levels after cessation of Statin medication other causes of elevated CK levels should be considered and investigated, including anti HMG-CR associated SAM [15]. Patients should be informed about the risk developing myopathy and symptoms. Despite the risk of SAM Statin therapy remains a useand powerful tool in cardiovascular risk reduction and in reducing cardiovascular morbidity and mortality. Their benefits considerably supersede their risk profile [9]. Our case emphasizes the need for clinicians to be aware of Statin associated necrotizing myopathy even after long term Statin treatment. The presented patient received uneventful Simvastatin therapy for a period of six years. In addition, we identified Colchicine as potential trigger of SAM in our patient based on its potentially competing metabolism with Statin. Fast recognition of SAM is mandatory to rescue renal function and avoid life threatening complications. The treatment of choice remains immediate cessation of Statin medication and supportive care for renal function. If recognized early enough, outcome is excellent.
We declare no conflicts of interest.