
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Research Article - (2024)Volume 14, Issue 1
Amount of e-waste is increasing tremendously over the years, from almost getting doubled each year. 40% e-waste consists of many metals and precious metals. The rapid growth in technology development is alarming, as a result more and more e-waste is generating. This leads to myriad problems for handling or management of e-waste. Traditional methods of disposal of e-waste such as landfilling, composting and incineration are major threat to the environment and life. In this study recovery of metals through hydro-metallurgical process such as thiosulfate (M1), iodide (M2) aqua-regia (M3) and thiourea (M4) leaching methods were compared in terms of economic feasibility, environmental impact and reagent reuse in order to find out a feasible leaching method using Analytic Hierarchy Process (AHP). The selection of feasible leaching method has been performed by applying the Saaty, ranking. From the final ranking, thiosulfate (M1) scored=0.09, iodide (M2)=0.16, aqua-regia (M3)=0.39 and thiourea (M4)=0.39. From the result M3 and M4 are the feasible method for recovery of heavy metals from e-waste.
Leaching methods; AHP technique; E-waste; Recovery; Heavy metals
E-waste is referred to the electrical and electronic equipment which have reached end of their useful life. In last 10 years utilization of hardware and electrical gear are increasing very drastically and simultaneously e-waste are also generating harshly. E-waste contains over 60% precious and nonmetals parts and many are valuable, some are hazardous, and some are both. High rate of e-waste generation could be a threat to the environmental pollution due to its hazardous components. Therefore, e-waste generating and management is a new emerging problem in the globe [1]. Some definitions from literature are:
European Waste from Electrical and Electronic Equipment (WEEE) directive
“Electrical or electronic equipment which is waste including all components, sub-assemblies and consumables, which are part of the product at the time of discarding.” [2]. Basel action network “E-waste encompasses a broad and growing range of electronic devices ranging from large household devices such as refrigerators, air conditioners, cell phones, personal stereos, and consumers electronics to computers which have been discarded by their users” [3]. E-waste contains a number of constituents which can be divided into 5 categories-ferrous metals, non-ferrous metals, glass, plastics and others. The first major constituent is metals and plastic are the second by weight. As per association of plastics manufacturing, the electronic and electrical products contain ferrous 38%, nonferrous 28%, glass 4%, plastics 19%, others 10%, and wood 1%, (Figure 1) [2]. Electronic and electrical products are converting in e-waste due to hasty progresses in technologies and more frequent replacement of these products by consumers [1]. E-waste is produced by mainly from institutions, household, and manufacturing sectors, etc., Disposal of e-waste is a major global and environmental concern. This waste has become the most tremendously growing segment of the municipal waste in world. The major problem lies as to how to carry out safe and efficient disposal of e-waste. This type of waste has been exponentially growing over the last many decades and accumulation may lead to biohazards. However, in up-to-date products contains up to 57 different elements. As per Kaya [4], the most complex structure in electronic and electrical item is present in printed circuit boards. Different categories of E-waste are presented in Figure 2.
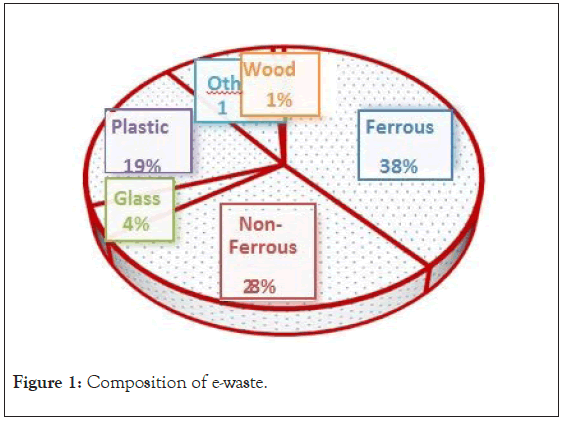
Figure 1: Composition of e-waste.
Figure 2: Sources of e-waste [5].
Electronic waste items contain numerous components that are hazardous and toxic in nature as presented in Figure 3. Therefore, they should be recycled by scientifically sustainable techniques; otherwise it may lead to devastating impact on life, environment and climate. If recycling is done properly, then waste electronic items provide immense value addition and drives great economic aspect [5,6]. One of the most used methods for E-waste disposal is landfilling, which poses several problems. The landfill sites release hazardous emissions. Mercury, cadmium and lead are the most toxic elements in the leachate [7]
Figure 3: Potential hazardous e-waste exposure.
As the amount of e-waste is increasing exponentially over the years and the practices of recycling them and disposing them off in landfills is a matter of environmental concern, it is necessary to find out a proper method for the extraction of metals from the e-waste as this will ace the recycling process. The process selected should be such that it is environmentally sound and economically viable. Therefore, the aim of this study is to find out the feasible leaching method for recovery of heavy metals from e-waste using analytic hierarchical process based on the criteria of economic feasibility, environmental impact and reagent reuse.
Literature Survey
Status of India in global scenario: The main reasons for the significant increment in e-waste generation are development of new technologies, human mentality, and very important is population. As per the report given in 2018 by Associated chambers of commerce and industry of India, 2 MT per annual e-waste is formed in India in 2018 and comes in top five countries in the world, after China, USA, Japan and Germany [7,8]. But according to published article in Times of India in 2020 by Mohan [9], India generating 3.2 MT per annual and third largest e-waste generating country in the world after China and USA which are generating e-waste 10.1 and 6.9 MT per annual respectively. As per Mohan [9], about 38% of global e-waste is generated by these three countries. On per capita scales, Europe ranks first (16.2 Kg/capita) and Oceania ranks second (13.3 Kg/ capita) followed by America (13.3 Kg/capita) while India percapita e-waste generation is much lower (2.4 kg/capita).
Indian scenario: In India major sixty five cities are generating >60% of the total e-waste, while, 10 states are generated 70% of the total e-waste [1]. Maharashtra, Gujarat, Tamil Nadu, Uttar Pradesh, West Bengal, Andhra Pradesh, Karnataka, Madhya Pradesh, and Delhi are the major states in which e-waste generation is very high. In comparison with the city wise e-waste generation, Mumbai is the top first city which generated 24% e-waste. Figure 3 shows state wise e-waste generation in India.
The main factors for growth of e-waste in India are discussed below:
• Consumer end: Discarding of e-waste is a large issue from consumer point of view. Basically, some e-wastes like computer peripherals are reused/recycled more in India compared to developed countries with resale and repair facilities. Still few e-wastes become obsolete when consumer loses interest.
• Market factors: Large software industries use cutting edge technologies, greater computing speed and improved efficiency, thus, it increases the rate of obsolescence. Further, with growth in standard of living, banks are providing affordable loans and dealers provide easy installment facilities [10].
City wise generation of e-waste is shown in Figure 4, amongst top 10 cities, Mumbai ranks 1st in generating e-waste. IT sector plays a big role for the growth of economy of India on one hand, whereas on the other it has led to the increase in the consumption of electronic components and a tremendous growth of e-waste in the country. It is necessary to develop comprehensive and robust mechanism for waste disposal before it becomes unmanageable [11].
Figure 4: Status of status of India in global scenario.
Recovery of heavy metals
Waste electrical and electronics equipment’s processing is very complex due to its heterogeneous composition [12]. Generally, two key steps such as pre-processing and end-processing involved in recovery of heavy metals. In pre-processing, the equipment’s that have expired are dismantled manually at collection sites. Then the components are tested and secluded from WEEE. Initially, housing cabinet, all wirings, and drive boards are separated [13]. After that mechanical processing is applied using hammer mills for cutting the e-waste in small pieces. Then metals, non-metals, plastic are separated using different separation techniques such as screening, magnetic, eddy current and density [13]. Final stage in end-processing in which metal extraction takes place by chemical processes via pyro-metallurgy, hydro-metallurgy, electro-metallurgy or biometallurgy techniques.
The metal portions separated from electronic waste through endprocessing and their combinations. The pyro-metallurgical and hydro-metallurgical methods are the major paths for processing of electronic waste. Apart from these routes/methods, there are other methods like electrochemical or electrometallurgical processes (for example, we have electro winning or electro refining) for certain metal separation and recovery. Every method has its advantages and disadvantages that must be taken into consideration for the selection of a fitting recycling process of metal recovery.
Hydro-metallurgy process
Hydrometallurgical process comprise of a sequence of acid or caustic leaching of e-waste [14]. We cannot completely extract precious metals by directly using pyro-metallurgical processing. Therefore, the residues of copper and nickel from smelters are send for further processing. In hydro-metallurgy process, heavy metals are separated from acid and caustic solutions through precipitation, solvent extraction, adsorption and ionexchange process [14]. Leaching method involves the reaction of solid components when exposed to leaching agent. In these method precious metals targeted using chemicals and titration techniques from the solution of metal complex. Leaching agents such as thiosulfate, alkali, cyanide, and many acids are used. In acids, Hydrochloric Acid (HCl), Sulfuric Acid (H2SO4) and Nitric Acid (HNO3) are used. After using of leaching agents, target element is settled by gravity into the liner and sent for additional processing or recovery of the metals [15]. Leaching parameters are influenced by particle sizes, dose of solvents used, temperature, and the leaching time. The major challenge during the extraction of precious metals is formation of metal complexes with other metals. Though, chemical leaching is more preferable as compare to pyro-metallurgical processes. Further these processes do not release harmful gases and particulate matters in the environment. These processes consume very less energy [16]. Also, the recovery rates are higher and easy operation as compare to other process [17]. The advantages of hydro-metallurgical methods include, better control, more precision, and greener option as compared to the high operating temperatures of pyrometallurgy process. However, the main disadvantages of hydrometallurgical methods are time consuming, slow, and impact the recycling economy. During termination and subsequent steps there is risk of precious metal loss; hence total recovery of metals is affected [16].
Analytic hierarchy process
Analytical hierarchy process is a decision making tool developed by Thomas L. Saaty of the University of Pittsburgh [18]. AHP is used when has various measures are available for comparison [19,20].
Development of a hierarchy model
In this step, a three-level hierarchy model was developed as shown in Figure 5 to select the suitable leaching method using AHP. Criteria and sub-criteria are discussed in detail.
Figure 5: State wise e-waste generation in India.
Selection of criteria
Several leaching methods used for the recovery of metals from e-waste. The main criteria that will assist in deciding the suitable process for heavy metal recovery are represented here, i.e., economic feasibility, environmental impact, and reagent reuse.
Economic Feasibility (EF)
Economic feasibility is the economic analysis of the process, which include all expenditure or investment during the process. If all the expenditure is in low cost, then the process is called as an economically feasible process.
Environmental Impact (EI)
Environmental impact refers to the impact on the ecosystem. A suitable method should not impact or harm the environment.
Reagent Reuse (RR)
In leaching process reagent is the heart of the process, therefore, it should be reused again and again. Regeneration of reagent is a good characteristic of economic and eco-friendly process.
Selection of sub-criteria
On the basis of criteria, six sub-criteria such as leaching rate, reagent cost, toxicity, safety, corrosiveness, and reagent regeneration were selected for all the four methods.
Leaching Rate (LR)
Leaching rate is related to the kinetics of a process i.e., how much heavy metal is recovered in a particular time. A suitable method should have high leaching rate.
Reagent Cost (RC)
Reagent cost is the cost of reagent used for recovery of 1 g of e-waste. A suitable method should have low reagent cost.
Toxicity (TO)
Toxicity is the degree at which the reagent can harm the environment (organism) and human beings. A suitable method should be less toxic to flora and fauna.
Safety (SF)
Safety includes the condition of being protected from unlikely caused danger, risk, or injury. A suitable method should be safe.
Corrosiveness (CO)
Corrosiveness means the ability of a reagent to cause corrosion. A suitable method should be non-toxic and non-corrosive in nature.
Regeneration (RG)
After the completion of one cycle of recovery, the reagent can be recovered and used again for subsequent cycles. If regenerated reagent recovers in good amount, then the process is considered as suitable method.
Alternative
Comparison was made between thiosulfate (M1), iodide (M2) aqua-regia (M3) and thiourea (M4) for recovery of heavy metals.
Pairwise comparison matrix
In AHP, pairwise comparisons are made to get exact ratio scale priorities. A pairwise comparison matrix is constructed for each level (criteria, sub-criteria and alternatives) and generates a matrix of relative rankings. For example, comparing LR to all six sub-criteria, i.e., LR, RC, TO, SF, CO, and RG.
Judgment for pairwise comparison
In this step, judgments are made on the basis of decision makers experience and knowledge (literature survey). Pairwise comparison has been done as per Table 1. After that all values were normalized in the matrix by summing each column and then dividing each element of the matrix by the sum of its respective column to obtain the normalized pair-wise matrix which is called as Priority Vector (PV).
| Value | Definition | Explanation |
|---|---|---|
| 1 | Identical value | Two requirements are of equal value |
| 3 | Slightly more value | Experience slightly favours one requirement over another |
| 5 | Strong value | Experience strongly favours one requirement over another |
| 7 | Very strong value | Experience very strongly favours one requirement over another |
| 9 | Extreme value | Experience Extreme value favours one requirement over another |
| 2,4,6,8 | Intermediate values | When compromise is needed |
| Reciprocals | Reciprocals for inverse comparison |
Table 1: Scale for pair-wise comparisons Saaty TL (1980).
Consistency verification
• For consistency verification, we multiply the value of criteria weights by each column of the pair-wise comparison matrix. Then we have to calculate the value of the weighted sum by summing the elements in each row and named as New Vector (NV).
• Maximum eigen value (ℷmax) was calculated by averaging the value of NV/PV.
• Consistency Index (CI) was calculated using equation (1);
• CI=(ℷmax–n)/(n–1) (1)
• Where n is the number of elements.
• Consistency Ratio (CR) was calculated using equation (2); CR=CI/RI (2)
Where RI = Random Index (Table 2). The value of CR < 0.10, only then the matrix is reasonably correct to make the decisions based on the AHP.
| n | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| RI | 0 | 0 | 0.58 | 0.9 | 1.12 | 1.24 | 1.32 | 1.41 | 1.45 | 1.49 |
Note: RI: Random Index.
• Finally, the criteria weights are used to decide the priority of each criterion and tell its percentage weightage when multiplied by 100.
Comparison of leaching methods from literature
Comparison of leaching methods was performed by literature survey which is summarized in Table 3.
| Criteria | EF | EI | RG | |||
|---|---|---|---|---|---|---|
| Sub-criteria | LR | RC | TO | SF | CO | RG |
| M1 | Medium | Medium | Low | High | Least corrosive | Very low |
| M2 | High | Medium | Very low | High | Least corrosive | Low |
| M3 | Fairly high | Medium | Medium | Low | Highly corrosive | High |
| M4 | Fairly high | Low | Low | Medium | Fairly corrosive | Medium |
| Priority | M3 and M4 | M4 | M1 and M4 | M3 | M1 and M2 | M3 |
Overall suitability M3>M4>M1>M2
Note: EF: Economic Feasibility; EI: Environmental Impact; RG: Regeneration; LR: Leaching Rate; RC: Reagent Cost; TO: Toxicity; SF: Safety and CO: Corrosiveness.
Table 3: Comparison of leaching methods from literature survey.
Pairwise comparison and consitency test of criteria
Pairwise companion of criteria such as economic feasibility, environmental impact and reagent reuse were performed using Table 3 and the results are shown in Table 4. During the comparison, the first priority was given to economic feasibility, second to environmental impact and third to reagent reuse. From Table 4, the priority vectors for criteria (EF=0.65, EI=0.23 and RR=0.12) were obtained (Figures 6-8).
| EF | EI | RR | PV | NV | NV/PV | Consistency test | |
|---|---|---|---|---|---|---|---|
| EF | 1 | 3 | 5 | 0.65 | 1.94 | 3.007 | λmax=3.003 |
| EI | (1/3) | 1 | 2 | 0.23 | 0.69 | 3.002 | CI=0.0018 |
| RR | (1/5) | (1/2) | 1 | 0.12 | 0.36 | 3.001 | CR=0.0013 |
Note: EF: Economic Feasibility; EI: Environmental Impact; RR: Reagent Reuse; PV: Priority Vector and NV: New Vector.
Table 4: Pairwise comparison and consistency test of criteria.
Figure 6: City wise e-waste generation in India.
Figure 7: Metal extraction methods.
Figure 8: Diagram of hierarchical structure.
Pairwise comparison and consitency test of sub-criteria
Pairwise companion of sub-criteria such as leaching rate, reagent cost, toxicity, safety, corrosiveness, and reagent regeneration were performed using Tables 1 and 3. Results are shown in Table 5. During the comparison priority was set as per the literature. From the Table 5, the priority vectors for sub-criteria are LR=0.33, RC=0.67, TO=0.17, SF=0.83, CO=0.2, and RG=0.8.
| LR | RC | PV | NV | NV/PV | Consistency test | |
|---|---|---|---|---|---|---|
| LR | 1 | 1/2 | 0.33 | 0.67 | 2 | |
| RC | 2 | 1 | 0.67 | 1.33 | 2 | |
| TO | SF | PV | NV | NV/PV | ||
| TO | 1 | 1/5 | 0.17 | 0.33 | 2 | |
| SF | 5 | 1 | 0.83 | 1.67 | 2 | λmax=2 |
| CI=0 | ||||||
| CO | RG | PV | NV | NV/PV | RI=0 | |
| CO | 1 | 1/4 | 0.2 | 0.4 | 2 | CR=0 |
| RG | 4 | 1 | 0.8 | 1.6 | 2 |
Table 5: Pairwise comparison and consistency test of sub-criteria.
Pairwise comparison and consitency test of leaching methods (Alternatives)
Pairwise companion of leaching methods M1, M2, M3 and M4 with respect to Sub-criteria leaching rate, reagent cost, toxicity, safety, corrosiveness, and reagent regeneration were performed using Tables 1 and 3 and the results are shown in Table 6.
| For LR | M1 | M2 | M3 | M4 | PV | NV | NV/PV | Consistency test |
|---|---|---|---|---|---|---|---|---|
| M1 | 1 | (1/3) | (1/5) | (1/5) | 0.07 | 0.29 | 4.36 | |
| M2 | 3 | 1 | (1/4) | (1/4) | 0.13 | 0.56 | 4.29 | λmax =4.16 |
| M3 | 5 | 4 | 1 | 2 | 0.54 | 2.13 | 3.95 | CI=0.054 |
| M4 | 5 | 4 | (1/2) | 1 | 0.37 | 1.49 | 4.06 | RI=0.90 |
| CR=0.061 | ||||||||
| For RC | M1 | M2 | M3 | M4 | PV | NV | NV/PV | Consistency test |
| M1 | 1 | (1/3) | (1/3) | (1/5) | 0.07 | 0.31 | 4.14 | |
| M2 | 3 | 1 | (1/3) | (1/4) | 0.14 | 0.58 | 4.05 | λmax =4.201 |
| M3 | 3 | 3 | 1 | (1/4) | 0.23 | 1.03 | 4.4 | CI=0.086 |
| M4 | 5 | 4 | 4 | 1 | 0.55 | 2.43 | 4.44 | RI=0.90 |
| CR=0.095 | ||||||||
| For TO | M1 | M2 | M3 | M4 | PV | NV | NV/PV | Consistency test |
| M1 | 1 | (4/5) | 4 | (1/3) | 0.22 | 0.89 | 4.1 | |
| M2 | (5/4) | 1 | 5 | (5/4) | 0.35 | 1.42 | 4.12 | λmax =4.15 |
| M3 | (1/4) | (1/5) | 1 | (1/4) | 0.07 | 0.28 | 4.12 | CI=0.051 |
| M4 | 3 | (4/5) | 4 | 1 | 0.37 | 1.57 | 4.28 | RI=0.90 |
| CR=0.057 | ||||||||
| For SF | M1 | M2 | M3 | M4 | PV | NV | NV/PV | Consistency test |
| M1 | 1 | (1/3) | (1/4) | (4/5) | 0.1 | 0.42 | 4.1 | |
| M2 | 3 | 1 | (1/4) | (4/5) | 0.18 | 0.75 | 4.11 | λmax =4.18 |
| M3 | 4 | 4 | 1 | 5 | 0.57 | 2.43 | 4.25 | CI=0.061 |
| M4 | (5/4) | (5/4) | (1/5) | 1 | 0.14 | 0.61 | 4.28 | RI=0.90 |
| CR=0.067 | ||||||||
| For CO | M1 | M2 | M3 | M4 | PV | NV | NV/PV | Consistency test |
| M1 | 1 | (1/3) | 5 | 4 | 0.3 | 1.32 | 4.4 | |
| M2 | 3 | 1 | 5 | 4 | 0.51 | 2.25 | 4.46 | λmax =4.25 |
| M3 | (1/5) | (1/5) | 1 | (1/3) | 0.07 | 0.27 | 4.09 | CI=0.085 |
| M4 | (1/4) | (1/4) | 3 | 1 | 0.13 | 0.53 | 4.08 | RI=0.90 |
| CR=0.094 | ||||||||
| For RG | M1 | M2 | M3 | M4 | PV | NV | NV/PV | Consistency test |
| M1 | 1 | (1/3) | (1/7) | (1/5) | 0.06 | 0.23 | 4.06 | |
| M2 | 3 | 1 | (1/6) | (1/4) | 0.11 | 0.44 | 4.05 | λmax=4.17 |
| M3 | 7 | 6 | 1 | 3 | 0.56 | 2.42 | 4.31 | CI=0.059 |
| M4 | 5 | 4 | (1/3) | 1 | 0.27 | 1.17 | 4.3 | RI=0.90 |
| CR=0.065 | ||||||||
Table 6: Pairwise comparison and consistency test of leaching methods.
Selection of feasible leaching method
The selection of feasible leaching method is done based on the final ranking of the alternatives using AHP technique. Priority vector from Tables 4-7 used for the selection of suitable leaching method. Value of PV alternatives and sub-criteria were multiplied and results are shown in Table 8. Similarly, the results obtained in Table 8 were further multiplied by PV value of criteria to get final scores of each method and tabulated in Table 9. It can be observed from Table 9 that thiosulfate (M1) scored=0.39, iodide (M2)=0.16, aqua-regia (M3)=0.39 and thiourea (M4)=0.39. As per Saaty the alternative with the highest priority would be the most suitable method. So, alternative with highest priority is aqua-regia (M3) and thiourea (M4) which is highest compared to other alternatives. Hence having worked out the AHP technique, the aqua-regia (M3) and thiourea (M4) method is judged to be the most suitable method for the recovery of heavy metals from e-waste.
| PV values | LR (0.33) | RC (0.67) | TO (0.16) | SF (0.88) | CO (0.2) | RG (0.8) |
|---|---|---|---|---|---|---|
| M1 | 0.06 | 0.07 | 0.22 | 0.1 | 0.3 | 0.05 |
| M2 | 0.13 | 0.14 | 0.35 | 0.18 | 0.51 | 0.1 |
| M3 | 0.54 | 0.23 | 0.07 | 0.57 | 0.07 | 0.56 |
| M4 | 0.36 | 0.55 | 0.37 | 0.14 | 0.13 | 0.27 |
| PV for LR and RC | PV for TO and SF | PV for CO and RG | ||||
| M1 | 0.06 × 0.33+0.67 × 0.07=0.069 | 0.22 × 0.16+0.88 × 0.1=0.123 | 0.3 × 0.2+0.8 × 0.05=0.105 | |||
| M2 | 0.13 × 0.33+0.67 × 0.14=0.137 | 0.35 × 0.16+0.88 × 0.18=0.214 | 0.51 × 0.2+0.8 × 0.10=0.189 | |||
| M3 | 0.54 × 0.33+0.67 × 0.23=0.33 | 0.07 × 0.16+0.88 × 0.57=0.513 | 0.07 × 0.2+0.8 × 0.56=0.464 | |||
| M4 | 0.36 × 0.33+0.67 × 0.55=0.490 | 0.37 × 0.16+0.88 × 0.14=0.182 | 0.13 × 0.2+0.8 × 0.27=0.245 |
Table 7: Overall priority vectors for alternatives with respect to sub-criteria
| EF (0.65) | RI (0.23) | RG (0.12) | |
|---|---|---|---|
| M1 | 0.069 | 0.123 | 0.105 |
| M2 | 0.137 | 0.214 | 0.189 |
| M3 | 0.332 | 0.513 | 0.464 |
| M4 | 0.49 | 0.182 | 0.245 |
Table 8: Overall priority vectors for alternatives with respect to criteria.
| PV for EF, EI and RR | Final PV | |
|---|---|---|
| M1 | 0.069 × 0.65+0.23 × 0.123+0.12 × 0.105 | 0.09 |
| M2 | 0.137 × 0.65+0.23 × 0.214+0.12 × 0.189 | 0.16 |
| M3 | 0.332 × 0.65+0.23 × 0.513+0.12 × 0.464 | 0.39 |
| M4 | 0.490 × 0.65+0.23 × 0.182+0.12 × 0.245 | 0.39 |
Table 9: Final PV of each method.
Hydrometallurgical methods have been effectively used for metals recovery from e-waste around the world, owing to its simplicity and controlled process with high recovery rates at relatively low costs, and a variety of studies using various hydrometallurgical methods for metals recovery from e-waste has been published every year. This study provides an up-todate review of the hydrometallurgical recovery of metals from e-waste and gives perspectives of this particular area, which is expected to provide an insight for the selection of suitable hydrometallurgical leaching and purification methods that would be the future focuses of this area. Further, systematic approach, analytic hierarchy process was used for selection of feasible (suitable) leaching method for heavy metal recovery. The overall priorities of the sub criteria with respect to the criteria for each method are given in this study.
More attention should be paid on developing novel leaching methods using environmentally benign and easily recycled lixiviant and oxidant. Complex metal compositions present in e-waste, therefore, simplify the downstream purification process, careful leaching should be considered. Suitable methods for the purification and recovery of metals from leaching solution should be decided based on the specific leaching solution.
The authors are thankful to University School of Chemical Technology, Guru Gobind Singh Indraprastha University, New Delhi, India, for providing facilities to carry out the research work in the concerned area.
The author declared no conflict of interest.
TAll data, models, and code generated or used during the study appear in the submitted article.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Minocha G, Khandegar V, Acharya S (2024) Selection of Feasible Leaching Method for Recovery of Heavy Metals from E-Waste Using Analytic Hierarchy Approach. J Clin Toxicol. 14:558.
Received: 06-Feb-2024, Manuscript No. JCT-24-29495; Editor assigned: 08-Feb-2024, Pre QC No. JCT-24-29495 (PQ); Reviewed: 22-Feb-2024 Revised: 29-Feb-2024, Manuscript No. JCT-24-29495 (R); Published: 07-Mar-2024 , DOI: 10.35248/2161-0495.24.14.558
Copyright: © 2024 Minocha G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.