Journal of Nanomedicine & Biotherapeutic Discovery
Open Access
ISSN: 2155-983X
ISSN: 2155-983X
Research Article - (2016) Volume 6, Issue 2
Introduction: Cigarette smoke has been proven to be injurious to both oral cavity and internal body environment. Passive smokers could also suffer from smoke. Saliva, the first biological fluid which encounters cigarette smoke, contains antioxidant defense system to reduce the toxic effects of cigarette smoke. Objectives: The aim was investigating salivary
antioxidants
of passive smokers as compared to non-smokers. Method: Un-stimulated whole saliva samples, obtained from passive smokers and non-smokers, were centrifuged and stored at -70°C. The antioxidant power was then measure using various methods. Results: No statistically significant difference in antioxidant capacity, total phenol, and radical scavenging activity of saliva between studied groups was found. However, concentration of uric acid, the important antioxidant of saliva, was decreased dramatically in passive smokers. Conclusions: It was suggested that measurement of antioxidants in salivary fluid could be a useful noninvasive method to investigate abnormalities related to oral cavity and
gastrointestinal tract.
Keywords: Carcinogens; Cigarette; Secondhand smoke; Salivary antioxidants
Passive smoking, chiefly among young children, may cause serious health problems according to a report by World Health Organization, WHO [1]. It is estimated that almost one billion grown up people smoke worldwide and at least 700 million children live with them. Clearly, children are the most vulnerable group who are in danger of passive smoking [2]. Many researchers have studied the deleterious effect of passive smoking on respiratory disorders [3,4], snoring or sleep fragmentation in children [5] and infants [6]. It is believed that the prevalence of childhood leukemia in children who live with smoker parents is a consequence of passive smoking [7]. On the other hand, there are only a few works showing the relationship between general oral health or formation of dental caries and passive smoking in preschool [8], elementary school children [9,10] and adolescent [11,12].
Human whole saliva is a complex mixture of components in many aspects similar to other body fluids [13]. Saliva is secreted by three paired major salivary glands and hundreds of minor salivary glands which is located below mucosal surfaces of the mouth [14,15].
Saliva is composed of 99% water together with different locally produced proteins and enzymes, glycoproteins, electrolytes, epithelial and immune cells, microorganisms, bronchial products, with some other biochemical such as antioxidants [16,17]. Consequently, salivary fluid could be considered for use as a suitable biomarker for oral disorders [18,19] as well as some systemic disease [20]. It has been shown that saliva can also reflect the relationship between oral hygiene and some chronic systemic diseases [21]. However, the antioxidant capacity and reducing power of saliva may be reduced due to various factors as well as in vitro exposure to cigarette smoke which could significantly decrease biological activity of some enzymes, both in plasma and in saliva [22-24].
Chemicals and equipments
2,4,6-Tripyridyl-s-triazine (TPTZ), 2,2-Diphenyl-2-Picrylhydrazyl Hydrate (DPPH), sodium acetate, sodium carbonate, acetic acid, Hydrochloric Acid (HCl), gallic acid, ferric chloride, Folin-Ciocalteu’s phenol reagent and ferrous sulphate, were purchased from Sigma representative in Iran. Uric acid kit was purchased from Pars Azmoon Company in Iran. All other chemicals and solvents were of reagent grade and used as supplied.
Participants
The participants were 45 male passive smokers and 45 male nonsmokers. A passive smoker was one who lived with a person smoking 15-20 cigarettes per day for at least 5 years. All subjects were university students 18-22 years old with healthy teeth and gums and did not suffer from any internal or genetic disease. The students lived in the dormitory of University of Guilan with the same diet and similar living conditions. They were informed about the nature of the study and filled a questionnaire about their health background and various aspects of their life style.
Saliva collection and storage
Un-stimulated saliva samples were collected from all volunteers after eight hours of fasting around 8-9 AM. Participants were instructed to rinse their mouth with distilled water and keep their saliva for exactly 3.0 minutes. The un-stimulated saliva was then collected in clean, dry and sterile pre-weighted tubes [25]. And placed in ice then centrifuged immediately at 9000 rpm for 12 minutes at 4°C and cell debris was rid of. The supernatant was stored at -70°C until tests were performed.
DPPH radical scavenging assay
This method is based on the reduction of DPPH alcoholic solution in the presence of hydrogen donating antioxidants, especially phenol components. The procedure we used in this study was a modification of the method described in Bompadre et al. [26]. In this chemical assay, DPPH radical scavenging activity of saliva samples against stable DPPH° was measured spectrophotometrically. The reduction of DPPH radical leads to a color change from deep-violet to light-yellow. The color change was measured at 517 nm using a UV/visible spectrophotometer (Ultrospec 3000, Pharmacia Biotech™, Sweden). Briefly, 1500 μl of freshly prepared DPPH° (1 × 10−4 M) solution in methanol (made in darkness) was added to 50 μl of centrifuged saliva in test tubes and mixed. The samples were kept in darkness for 30 min at room temperature and the absorption was then measured at 517 nm (Ac) against methanol as blank. The absorbance of the methanol solution of DPPH° was also measured as control (Ac). All experiments were carried out in at least duplicate and radical scavenging activity was calculated using the following relationship:
DPPH radical scavenging activity (%) = [(Ac – AS) /Ac] × 100
Total antioxidant capacity by FRAP
The total antioxidant activity of salivary samples was determined by Ferric Reducing Assay Power (FRAP) [27]. The FRAP reagent was prepared by mixing 300 ml of acetate buffer pH 3.6, 20 ml of FeCl3, and 10 ml of 2,4,6-tripyridyl-s-triazine (TPTZ) in 40 ml of HCl. The freshly prepared FRAP reagent (120 ml) as the stock solution was smoothly heated to 37 °C for 10 min. Then 3 ml of final FRAP reagent was mixed with 100 μL of centrifuged saliva for each individual sample and after 30 minutes absorbance was measured at 593 nm.
A standard curve was prepared using different concentrations (3.9-77 μM) of FeSO4·7H2O for calculating the total antioxidant capacity. The results were corrected for dilution and expressed in mol FeSO4/L.
Determination of uric acid in saliva
Salivary uric acid concentration was measured using an enzymatic kit obtained from Pars Azmoon. The principle of kit was based on following a pink chromophore, the final product of a couple of enzymatic reactions. In the first phase uricase produces H2O2, and in the second phase H2O2, 4-aminoantipyrine, and TOOS are used by peroxidase to produce indamines, the pink chromophore detectable at 546nm.
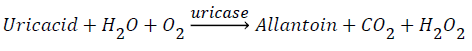
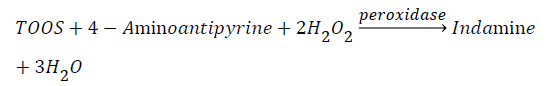
To this purposive, in the first phase, 20 μL of centrifuged saliva was added to 1000 μL of the first reagent of the kit, then it was shook at 25°C for 10 minutes. In the second phase, 250 μL of the second reagent of the kit was added to the solution, and this solution was shook for the second time at 25°C for 10 minutes and then absorbance was measured at 546 nm.
The standard solution was prepared by using 20 μL of the standard reagent instead of saliva sample.
Determination of total phenol content
Salivary total phenol concentration was measured by Goa method [28] using Folin-Ciocalteu’s phenol reagent. In practice, 100 μL of saliva was added to 200 μL of Folin-Ciocalteu’s phenol reagent and 2000 μL of de-ionized water and mixed well. After three minutes, 1000 μL sodium carbonate 20% was added in darkness. The final solution was kept in dark at room temperature for an hour. The absorbance of resulting solution was detected spectrophotometrically at 765 nm.
A standard curve was prepared using different concentrations (0.5-2.5 gr/lit) of gallic acid to which 2.5 gr/lit of gallic acid was provided by methanol 50%. Other concentrations were then prepared by diluting the primary stock for calculating the total phenol concentration of saliva samples. The results were expressed in gr/lit of total phenol.
Statistical analysis
Each assay was repeated at least duplicate and the results were presented as mean ± SD values. Statistical differences between individuals were considered by independent sample t-Test, p values less than 0.05 were considered as significant.
DPPH assay
The DPPH test measured the radical scavenging activity of saliva samples based on the change of DPPH radical to stable DPPH molecule by a reduction process on antioxidants.Results were expressed as percent of scavenging activity against DPPH radical. Table 1 compares the radical scavenging activity of saliva against DPPH• in passive smokers and nonsmokers. It is clear that no statistically significant difference existed in the activity of saliva against DPPH• free radicals among studied groups.
| Group | N | Mean | Standard deviation (SD) | P-value |
|---|---|---|---|---|
| Passive smoker | 45 | 10.39934 | 9.984317 | 0.395 |
| Non smoker | 45 | 11.77249 | 5.491011 |
Table 1: Antioxidant activity measured by DPPH test in saliva of passive smokers and nonsmokers.
FRAP test
The FRAP test is basically designed for quantitative measurement of the general capacity of biological samples in chelating and inactivation of metal ions especially ferric and ferrous ions. These are involved in the formation of highly reactive oxygen and nitrogen species (ROSs and RNSs). Table 2 depicts that the reducing power of saliva against ferric ions was the same in salivary fluids of nonsmokers and passive smokers.
| Group | N | Mean | Std. Deviation | P-value |
|---|---|---|---|---|
| Passive smoker | 45 | 0.1244782 | 0.006216801 | 0.564 |
| Non smoker | 45 | 0.1297401 | 0.007150724 |
Table 2: Antioxidant capacity of saliva samples measured by FRAP test.
Salivary uric acid
Uric acid and its salts are the final products of purine metabolism. Uric acid is the most important antioxidant in human saliva. Its increased concentration in the body can cause some disorders including goat and urinary stones. It has been reported that uric acid is responsible for about 70% of the total capacity of salivary antioxidant defense system this is by the fact that only 10% of the whole salivary antioxidant capacity is the protective share of lipid-soluble antioxidants [29-31]. Uric acid is totally water soluble and induces its antioxidant operation considerably faster than fat soluble antioxidants [32,33]. The final result of our study revealed that there was a dramatic decline in the amount of uric acid in passive smokers as compared to nonsmokers. The mean amount of uric acid in passive smokers was 2.9902 mg/dl whereas this amount for nonsmoker subjects was 9.2278 mg/dl (Table 3). This is a significant decrease with mean value of 32.40%. The average amount of uric acid in our control studied group was almost 1.02 mg/dl higher than the reported group.
| Group | N | Mean | Std. Deviation | P-value |
|---|---|---|---|---|
| Passive smoker | 45 | 2.9902 | 2.44876 | 0.005 |
| Non smoker | 45 | 9.2278 | 2.99181 |
Table 3: Concentrations of uric acid the most important non-enzymatic antioxidant in saliva samples.
Total phenol
The total phenol test is a quantitative test for measuring the entire phenol present in any solution including saliva samples. Table 4 has compared the total phenol concentration in passive smokers and nonsmokers. It is clear that there was not statistically significant difference in the total phenol concentration among studied groups.
| Group | N | Mean | Std. Deviation | P-value |
|---|---|---|---|---|
| Passive smoker | 45 | 0.27148778 | 0.110141178 | 0.343 |
| Non smoker | 45 | 0.28607330 | 0.142464908 |
Table 4: Concentrations of total phenol in saliva samples.
Cigarette smoke contains more than 4000 chemicals, some of which are nicotine, ammonia, acrolein, phenols, acetaldehyde, benzopyrine, nitrogen oxides, carbon monoxide, polonium, radium and thorium [34]. Besides, cigarette smoke contains free radicals which are deleterious for various parts of the body. It is worth to indicate that just one cigarette puff contains 1014 free radicals in the tar phase and 1015 free radicals in the gas phase [35]. The relationship between DNA damage and tar phase free radicals after smoking has been described by Pryor and Stone [36]. These radicals are mostly quininehydroquinone which is not highly reactive [36]. On the other hand, it is known that gas phase free radicals are generally more reactive than those in the tar phase [37]. Tobacco smoke can alter the antioxidant power of saliva. However, the nature of alternations is not known with certain [38].
The results of this study showed statistical differences in the level of salivary uric acid between passive smokers compared to nonsmokers. However, it was observed that radical scavenging activity, total antioxidant capacity and total phenolic content in saliva samples did not vary statistically between the two groups. On the other hand, contrasting results were found for enzymatic antioxidants including peroxidase, superoxide dismutase and catalase within our group (in a parallel study, the results to be published in due course). It has been found that activity of antioxidant enzymes were significantly lower in salivary fluid of passive smokers compared to nonsmoker group. Radical scavenging activity of saliva, total antioxidant capacity and total phenolic content in saliva samples could be related to the fact that passive smokers are in-directly exposed to smoke. However, they partially inhale cigarette smoke in some degrees, so their salivary antioxidant defense system shows a degree of variations in terms of uric acid and enzymatic antioxidants. This indicates the sensitivity of uric acid content and enzymatic activity of saliva which is an interesting result, suggesting these factors could be used as salivary markers of smoke intoxication. It also explains the higher share of uric acid in salivary antioxidant defense system that is of great non-invasive diagnostic value for passive smokers.
Passive smoking can be significantly dangerous to fetus through decreasing the activity of defense system. It has been reported that cigarette smoking during pregnancy can cause a number of adverse perinatal outcome. The hazards of smoking during pregnancy on oxidative damage and antioxidant defense in matched samples of maternal blood and cord blood has been reported [39]. In support of our results, it is shown that tobacco smoke enhances lipid peroxidation and lowers antioxidant potential in the plasma of pregnant women and umbilical cord blood. Maternal cigarette smoking has also been reported to be associated with evidence of chronically increased resistances in the uterine, umbilical and fetal middle cerebral arteries [40].
On the other hand, a high daily intake of vitamins and antioxidants could offer protective effect against biochemical and molecular processes that lead to cancer, cardiovascular disease and respiratory illness. For example, a Mediterranean diet is comprised of a number of compounds that could alter certain outcomes related to smoking [41]. According to a research in 2014, vitamin C supplementation, an antioxidant stimulus, could reduce the adverse effect of chronic passive smoking which is a pro-oxidant stimulus [42].
The effect of passive smoking on children who were exposed to at least 10 cigarette per day for at least last 1 year in their house has shown similar results to our obtained results here [43]. They reported that total antioxidative response of plasma was significantly lower, total peroxide level of plasma was significantly higher and mean oxidative stress index value was significantly higher in children exposed to passive smoking as compared to those not exposed group. Passive smoking may also affect DAN leading to mutation or harmful alternations in the next generation. Investigating the level of damage to DNA, a research study has measured the serum level of malondialdehyde (MDA) and activity of glutathione peroxidase (GSHPx) in erythrocytes of highly (<20 cigarette per day), medium (<10 cigarette per day) and non-exposed children. They found that the exposed children had significantly (P < 0.001) higher MDA level and significant (P < 0.001) decrease in the level of GSH-Px and tocopherol fractions compared with controls. It was concluded that exposure to cigarette smoke is associated with an increase in the level of oxidants and a simultaneous decrease in the level of antioxidants in the children's blood. This oxidant–antioxidant imbalance is one of the mechanisms leading to DNA damage detected in lymphocytes of ETSexposed children [44]. They concluded that environmental exposure to smoking is associated to serious damage to DNA.
Based on the results obtained in this study, it was found that the effect of passive smoking on the levels of salivary non-enzymatic antioxidants is different. The radical scavenging activity of saliva samples against stable DPPH radical, the general capacity of saliva to chelate and inactivate metal ions, and the total phenol concentration of saliva samples were not significantly altered due to passive smoking. However, the most important water soluble salivary antioxidant (i.e., uric acid) was significantly affected by passive smoking. We suppose that passive smokers are also at the risk of oxidative attack and that some antioxidants, including uric acid could be used as salivary markers of exposure to cigarette smoke in people living/working with heavy smokers. Fortunately during the last few years public understating of passive smoking hazards has highly improved. In many countries, it is an offence to smoke in a public place and specially in vehicle carrying anyone under the age of 18. In conclusion, according to the results from present study and studies previously done by our group and other groups, it is concluded that people who are exposed to passive smoking are exposed to oxidative stress, implicated in the etiopathogenesis of many disorders and even genetic mutation.
“The authors declare no conflict of interest”.