Chemotherapy: Open Access
Open Access
ISSN: 2167-7700
ISSN: 2167-7700
Research Article - (2022)Volume 10, Issue 5
Saikosaponins A (SSA) is one bioactive triterpenoid glycoside isolated from traditional Chinese herb Radix Bupleuri, and exerts antitumor effects in various types of human cancer cells. However, so far, there has been no any study concerning the effect of SSA on human Osteo Sarcoma (OS) cells. In this study, we presented new insights on the antitumor effects of SSA in human OS MG-63 cells. The CCK-8 assay and clone formation assay showed that SSA significantly inhibited MG-63 cell proliferation in a dose- and time- dependent manner. The typical morphology changes and results of Annexin V/PI double staining indicated that SSA induced apoptosis occurred in MG-63 cells. Furthermore, we also proved that SSA had the ability to suppress the migration and invasion of MG-63 cells. The western blotting results suggested that SSA increased the expression of Bax and cleaved-caspase 3, decreased expression of Bcl-2, and induced apoptosis via inactivation of PI3K/AKT signaling pathway. In conclusion, these findings reveal that SSA possesses significant antitumor effects on MG-63 cells and may serve as a promising therapeutic agent for the treatment of human OS.
Saikosaponins A; Osteosarcoma; Proliferation; Apoptosis; PI3K/AKT pathway
Osteo Sarcoma (OS) is a primary malignant bone tumor, which mainly arises within the metaphysis of long bones and occurs in children and adolescents [1]. OS is characterized by the high tendency of distant metastasis. The lung is the most common metastatic site and nearly 25% patients present with metastatic disease [2]. With the development of new adjuvant chemotherapy and surgery technology, the 5-year survival rate has been improved. Unfortunately, for patients with metastasis and chemotherapy resistance, the survival rates are still only 15% to 30% [3]. Therefore, it is urgent to study effective and safe drugs for the treatment of OS.
Natural products provide lead compounds for the development of novel drugs. Many bioactive natural agents have been utilized as chemotherapeutic drugs to suppress or prevent malignant tumors progression [4]. Natural product-originated drugs have a series of advantages, including strong anti-cancer efficacies and low side effects [4,5]. Radix Bupleuri (RB), known as one traditional chinese medicine, is the dry root of Bupleurum chinense DC and Bupleurum scorzonerifolium Willd [6]. Triterpenoid saponins are the main active components of RB, which exhibit various effects including anti-inflammatory, antiviral, antiepileptic, antifibrotic, and neuromodulatory [7]. Saikosaponins A (SSA) is one triterpenoid glycoside, which is the major bioactive constituent of triterpenoid saponins. It has been reported that SSA possessed antitumor activities in hepatoma cells, breast cancer cells, cervical cancer cells, lung cancer cells and neuroblastoma cells [8-11]. However, the effect of SSA on human osteosarcoma cells remains unclear. In the present study, we attempted to further investigate the biological roles of SSA and detected the effect of SSA on human OS MG-63 cells.
SSA (purity>98%) was purchased from Yuanye Biotech Company (Shanghai, China). RPMI 1640 medium and Fetal Bovine Serum (FBS) were obtained from Gibco (Grand Island, USA). Penicillin and streptomycin were from Solarbio (Beijing, China). Cell Counting Kit-8 (CCK-8) was from Dojindo Laboratories (Kumamoto, Japan). Annexin V/propidium iodide (PI) Apoptosis kit, bicinchoninic acid (BCA) protein assay kit and RIPA lysis buffer were all from Beyotime Biotech Co., Ltd. (Suzhou, China). Anti-PI3K (#4255, 1:1000), anti-AKT (#9272, 1:1000), anti-phospho-AKT (#4060, 1:500) and anti-phospho-GSK-3β (#5558, 1:500) were obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA). Anti-Bax (#BM3964, 1:1000), anti-Bcl-2 (#BM0200, 1:1000), anti-pro-caspase-3 (#BM4340, 1:500), anti-cleaved caspase-3 (#BA3592, 1:1000), β-action (#BM0627, 1:1000) and goat anti-rabbit/mouse secondary antibodies (#BA1056, 1:1000) were all purchased from Boster Biotechnology Co., Ltd. (Wuhan, China).
Cell culture
Human OS MG-63 cells were purchased from the Cell Bank of the Chinese Academy of Science (Shanghai, China). The cells were negative for bacteria, yeast, fungi, and mycoplasma. As our previous report [12], MG-63 cells were cultured in complete medium containing RPMI 1640 medium added with 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin. The culture environment was maintained at 37℃ with 5% CO2 and humidified air. All procedures were carried out by one single person, strictly adhere to sterile practices.
Cell viability assay
Cell viability was analyzed by CCK-8 assay. MG-63 cells were seeded in 96-well plates (1 × 104 cells/well) and cultured with complete medium for 24 hrs. Then the medium of each well was changed and cells were treated with different concentrations of SSA (20,40,80 μM) for 24,48 and 72 hours. After that, the cells in per well were incubated with 10 μl CCK-8 solution at 37℃ for 2 hrs. The absorbance at 450 nm was measured by a micro plate reader (Bio-Rad 680, Hercules, CA, USA).
Clone assay
MG-63 cells were seeded in 6-well plates (500 cells/well) and treated with various concentrations of SSA (0, 20, 40, 80 μM) for 48 hrs. Then the medium were replaced with complete medium and the cells were cultured for another 10 days. After that, the cells were stained with crystal violet for 10 min. The colonies were counted and photographed using a digital camera (Canon 600D, Japan). The rate of clone formation was calculated as follows: clone formation rate (%)=(colony number/500) × 100 [13].
Cell morphology observation
MG-63 cells were treated with various concentrations of SSA (0, 20, 40, 80 μM) for 48hrs. An inverted phase contrast microscope (Olympus CX23, Japan) was used to observe the changes of cell morphology (magnification, 200 ×). The photos were captured by a digital camera.
Cell apoptosis assay
The apoptosis of MG-63 cells was detected by Annexin V/PI double staining following a flow cytometry. MG-63 cells were treated with various concentrations of SSA (0, 20, 40, 80 μM) for 24 hrs. Then the cells were washed with cold Phosphate Buffer Saline (PBS) for twice and re suspended at a density of 1 × 106 cells/ml in 1× binding buffer. After that, 500 μl cell suspension was incubated with 5 μl Annexin V and 5 μl PI for 15 min at room temperature in dark. Cell samples were maintained on ice in dark and analyzed by a flow cytometry (BD FACScan, San Diego, CA, USA). Apoptosis ratio was calculated using FlowJo 10.0 software (Tree Star, Inc., USA).
Wound healing assay
MG-63 cells were seeded on 6-well plates and cultured to 80-90% confluence. A 10 μl sterile pipette tip was used to scratch the monolayer cell and create vertical lines. The unattached cells were removed with PBS. Lines with similar width were chosen and then the cells in each well were treated with 40 μM SSA for 24, 48 and 72 hrs. The migrating cells were observed by an inverted microscope and photographed using a digital camera.
Cell invasion assay
The cell invasion assay was performed in a transwell system (Corning, New York, USA) incorporated 6.5mm diameter inserts with polycarbonate filter of 8-μm- pore sizes. Before cell seeding, the membranes were coated with 50 μl matrigel (100μg/ml). Then 1 × 106 MG-63 cells were seeded in upper chamber and incubated with serum-free RPMI 1640 medium containing different concentration of SSA (0,20,40,80 μM). The lower wells were filled with 500μl medium supplemented with 20% FBS. After incubation for 24 hrs, the cells on the upper surface of chambers were wiped away by a cotton swab, and the invasive cells attached to the lower surface were fixed with methanol and stained with 0.1% crystal violet. The images were captured under a microscope (magnification, 200 ×) in 10 random fields. The invasive cells were counted using Image J 1.46r software.
Western blot analysis
MG-63 cells were incubated with various concentrations of SSA (0, 20, 40, 80 μM) for 24 hrs. Subsequently, the cells were harvested and lysed in the RIPA protein extraction buffer on ice for 30 min. The BCA protein assay kit was used to detect the concentrations of extracted proteins. Western blotting assay was carried out as our previous report [12]. The protein bands were visualized by an electrochemiluminescence imaging system (ChemiScope Mini 3400, CLINX, Shanghai, China) and further quantified using Image J 1.46r software.
Statistical analysis
Each experiment was performed in triplicate. All data were expressed as mean ± Standard Deviation (SD) and calculated using Graph Pad Prism 5 software. One-way ANOVA followed with Turkey’s post hot test was conducted to analyze the data. P<0.05 was considered statistically significant different.
SSA inhibits proliferation and colony formation of MG-63 cells
The effects of SSA on proliferation of MG-63 cells were investigated by CCK-8 assay. As shown in Figure 1A, SSA could significantly inhibit the viability of MG-63 cells in a time- and concentration-dependent manner (P<0.05). When treatment with 80 μM SSA for 24, 48 and 72 hrs, the viability was reduced to 55.63 ± 4.24%, 42.36 ± 3.04% and 28.71 ± 3.53% respectively, compared with the control group (0 μM SSA). In addition, the longterm effect of SSA on MG-63 cells growth was evaluated by clone formation assay. The results showed that treatment with SSA for 48 hrs could inhibit the growth of MG-63 cells. The colony formation rate was significantly decreased in a dose-dependent manner, compared with the control group (P<0.05) (Figures 1A-1C).
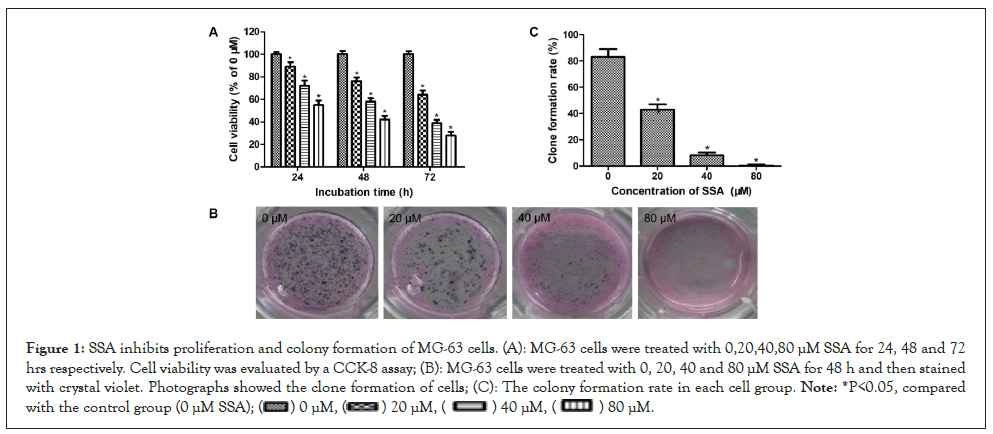
Figure 1: SSA inhibits proliferation and colony formation of MG-63 cells. (A): MG-63 cells were treated with 0,20,40,80 μM SSA for 24, 48 and 72 hrs respectively. Cell viability was evaluated by a CCK-8 assay; (B): MG-63 cells were treated with 0, 20, 40 and 80 μM SSA for 48 h and then stained with crystal violet. Photographs showed the clone formation of cells; (C): The colony formation rate in each cell group. Note: *P<0.05, compared with the control group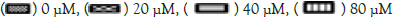
SSA induces morphology changes in MG-63 cells
The antigrowth effect of SSA was further confirmed by cellular morphology observation. After treatment with various concentrations of SSA (0, 20, 40, 80 μM) for 48 hrs, the morphology of MG-63 cells was observed by an inverted microscope. As shown in Figure 2, untreated normal cells attached to culture plates tightly and showed well-organized monolayer appearance. Whereas, SSA-treated cells showed remarkable changes, the cell membranes were broken and cells disconnected from each other. The total cell number was decreased and floating cells were increased. The changes were more obvious with increasing dose of SSA. These results indicated that SSA could induce cell death in MG-63 cells (Figure 2).
Figure 2: SSA induces morphology changes in MG-63 cells. MG-63 cells were treated with 0,20,40,80 μM SSA for 48 hrs. The changes of cell morphology were observed by an inverted microscope. Scale bar=50 μm.
SSA induces apoptosis of MG-63 cells
Further experiments were performed to assess whether the anticancer activity of SSA on MG-63 cells was associated with apoptosis. The apoptotic ratio was evaluated by Annexin V/PI double staining following a flow cytometry assay. The results indicated that the percentage of apoptotic cells significantly increased after SSA treatment. SSA could induce apoptosis of MG-63 cells in a dose-dependent manner (Figures 3A and 3B). To confirm this, the expressions of apoptosis-related proteins were detected by western blotting. After SSA treatment, the expressions of Bax and cleaved-caspase 3 increased significantly, and the expressions of Bcl-2 and pro-caspase 3 decreased. The expression level changes were correlated with the SSA concentration (Figures 4A and 4B). These results suggested that SSA could inhibit growth of MG-63 cells through triggering apoptotic signaling pathway.
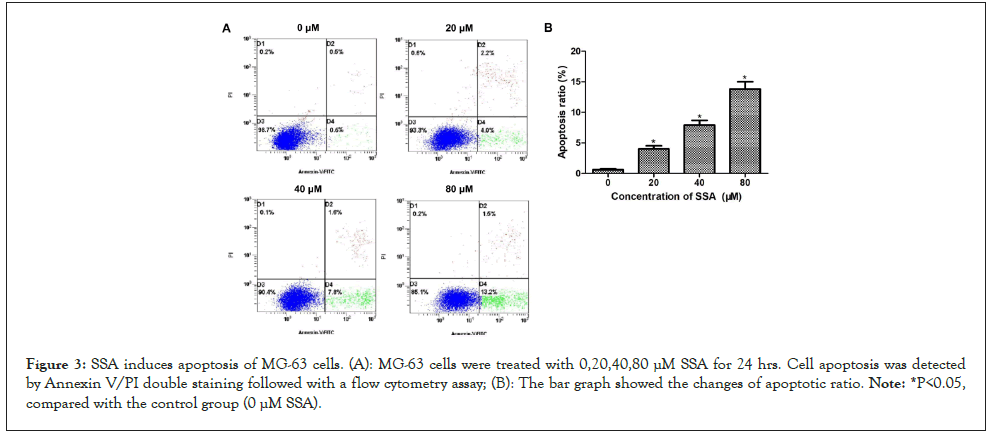
Figure 3: SSA induces apoptosis of MG-63 cells. (A): MG-63 cells were treated with 0,20,40,80 μM SSA for 24 hrs. Cell apoptosis was detected by Annexin V/PI double staining followed with a flow cytometry assay; (B): The bar graph showed the changes of apoptotic ratio. Note: *P<0.05, compared with the control group (0 μM SSA).
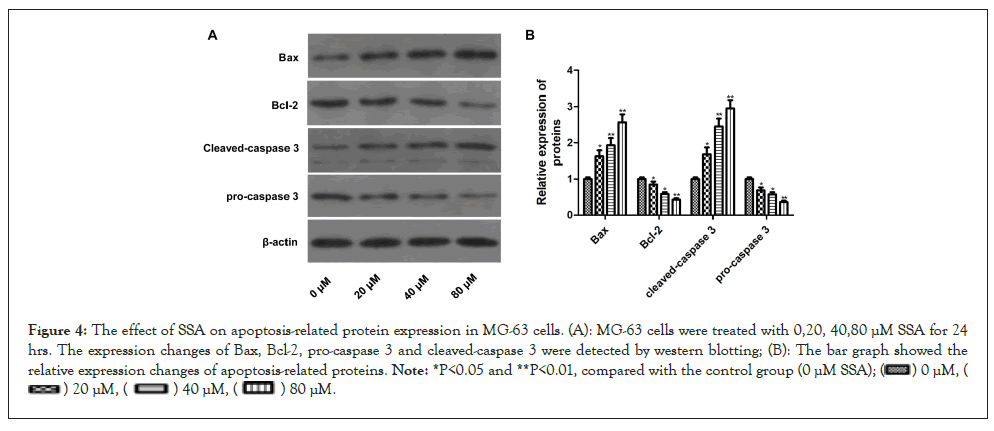
Figure 4: The effect of SSA on apoptosis-related protein expression in MG-63 cells. (A): MG-63 cells were treated with 0,20, 40,80 μM SSA for 24 hrs. The expression changes of Bax, Bcl-2, pro-caspase 3 and cleaved-caspase 3 were detected by western blotting; (B): The bar graph showed the relative expression changes of apoptosis-related proteins. Note: *P<0.05 and **P<0.01, compared with the control group (0 μM SSA);
SSA inhibits migration and invasion of MG-63 cells
Wound healing assay and trans well invasion assay were carried out to assess the effects of SSA on migration and invasion of MG-63 cells. In wound healing assay, MG-63 cells in the control group (0 μM SSA) possessed good in vitro migration ability. The scratched lines disappeared gradually with time increasing. After SSA treatment, the widths of wounds nearly didn’t change and the lines still existed after 72 hrs (Figure 5). In transwell invasion assay, SSA dose-dependently reduces the number of invaded cells (Figures 6A and 6B). These results indicated that SSA could inhibit the migration and invasion of MG-63 cells.

Figure 5: SSA inhibits migration of MG-63 cells. MG-63 cells were treated with 0 or 40 μM SSA for 24, 48 and 72 hrs respectively. The migration ability of cells was assessed by wound healing assay. The pictures showed the widths of scratched lines at different time points.
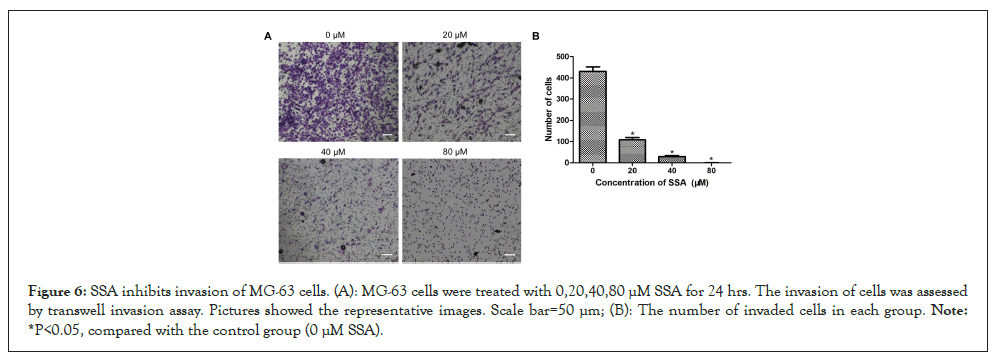
Figure 6: SSA inhibits invasion of MG-63 cells. (A): MG-63 cells were treated with 0,20,40,80 μM SSA for 24 hrs. The invasion of cells was assessed by transwell invasion assay. Pictures showed the representative images. Scale bar=50 μm; (B): The number of invaded cells in each group. Note: *P<0.05, compared with the control group (0 μM SSA).
SSA inactivates PI3K/AKT signaling pathway in MG-63 cells
Previous studies have demonstrated that the PI3K/AKT signaling pathway play an important role in apoptosis of human OS cells [14]. To explore the molecular mechanism of SSA-induced apoptosis in MG-63 cells, we detected the effect of SSA on PI3K/AKT pathway by western blotting assay. It was shown that the protein expression levels of p-AKT and p-GSK-3β were significantly decreased in SSA-treated group compared with the control group. Whereas, the expressions of total PI3K and AKT didn’t change. Therefore, the ratio of p/t-AKT was markedly decreased (Figures 7A and 7B). These results suggested that SSA could suppress the activation of PI3K/AKT signaling in MG-63 cells.
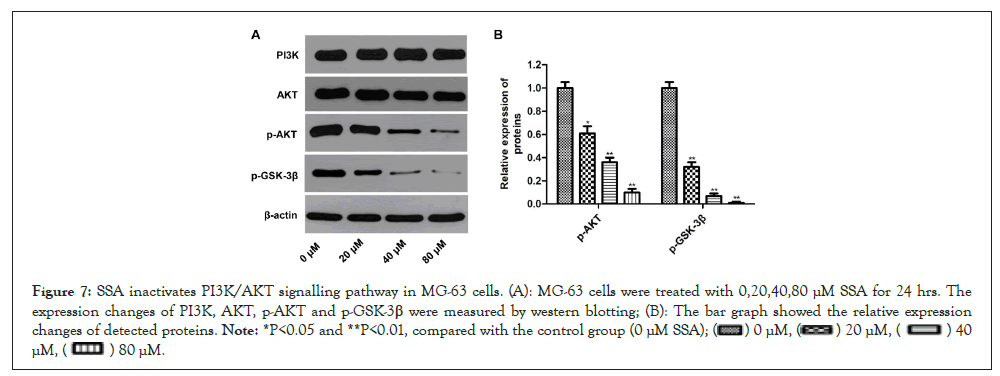
Figure 7: SSA inactivates PI3K/AKT signalling pathway in MG-63 cells. (A): MG-63 cells were treated with 0,20,40,80 μM SSA for 24 hrs. The expression changes of PI3K, AKT, p-AKT and p-GSK-3β were measured by western blotting; (B): The bar graph showed the relative expression changes of detected proteins. Note: *P<0.05 and **P<0.01, compared with the control group (0 μM SSA);
OS is the most common malignant bone tumor that mainly affects children and adolescents. Current standard treatment for OS including surgical resection and multiage chemotherapy, which only delay the progression of OS. The metastasis and mortality rates for OS patients remain high. For patients with metastatic disease or relapse, the survival rate is less than 30% [1,3]. Therefore, effective and safe therapeutic agents are needed to be discovered to improve the treatment of OS.
Natural products exhibit fewer side effects than chemical synthetic drugs, which have been used to treat various malignant tumors in the world [4,5]. SSA is isolated from traditional Chinese herb RB, and exerts potential antitumor activity in several types of cancer cells, such as breast cancer cells, colon carcinoma cells and neuroblastoma cells [8,10,15,16]. However, there is no any report about the effects of SSA against human OS cells. Here, we carried out a series of in vitro experiments to investigate the potential effects of SSA on human OS MG-63 cells. Results of CCK-8 and clone formation assay showed that SSA could inhibit the proliferation of MG-63 cells in a time- and concentration-dependent manner. The cell morphology changes indicated that SSA could induce cell death in MG-63 cells. Annexin V/PI staining and flow cytometry assay showed that SSA could induce apoptosis of MG-63 cells in a dose-dependent manner. Furthermore, wound healing assay and transwell invasion assay demonstrated that SSA could inhibit the migration and invasion of MG-63 cells.
Apoptosis also called programmed cell death, which plays a critical role in homeostasis of multicellular organisms. Evasion of apoptosis and uncontrolled growth may be the common features for cancer cells [17]. Apoptosis can be conducted via the mitochondria-dependent (intrinsic) pathway and death receptor-dependent (extrinsic) pathways. The intrinsic pathway is mediated by mitochondria dysfunction. Caspases-3 is a downstream executor that catalyzes the cleavage of cellular substrates [18]. The Bcl-2 family proteins, including ant apoptotic proteins Bcl-2 and proapoptotic proteins Bax, play a crucial role in the regulation of mitochondrial apoptotic pathway. Bax can induce the release of cytochrome C from the mitochondria. Bcl-2 can suppress releasing cytochrome C and activating caspase-dependent pathway. The balance of Bax and Bcl-2 determines the fate of cancer cells [19,20]. In our study, the expression ratio of Bax/Bcl-2 increased in SSA-treated MG-63 cells followed the upregulation of cleaved caspase-3. These findings indicated that mitochondrial pathway may be involved in SSA-induced apoptosis.
To further elucidate the molecular mechanism of SSA-induced apoptosis in MG-63 cells, the PI3K/AKT axis and its downstream signaling molecules were investigated. PI3K/AKT is a well-known signaling referring to cancer growth and metastasis [21,22]. Previous studies have reported the important role of PI3K/AKT axis in the proliferation, migration and apoptosis of OS cells. Activation of Akt can inhibit the mitochondrial pathway, subsequently stopping apoptosis. Inhibiting the phosphorylation of AKT can decrease the expression of downstream signaling factors, such as GSK-3β and caspase 3, thus induces cell apoptosis [23-26]. In this study, the expression levels of p-AKT and p-GSK-3β were significantly decreased in SSA-treated cells. These results indicated that SSA could induce MG-63 cells apoptosis via inactivation of PI3K/AKT signaling pathway.
Nevertheless, there were also some defects in our study. First, human OS includes several types of cell lines, thus the effects of SSA on other OS cells should be further investigated. Second, we only carried out in vitro experiments; the in vivo animal experiments should be further performed to verify the antitumor effects of SSA. In addition, the full molecular mechanism of SSA induced cell death also needs further study.
This study presented new insights on the antitumor effects of SSA in human OS MG-63 cells. SSA could inhibit the proliferation, migration and invasion of MG-63 cells. Mitochondrial pathway might be involved in SSA-induced apoptosis. The antitumor effect of SSA on OS might be mediated through inactivation of PI3K/AKT signaling pathway. These findings may indicate that SSA could be chose as a novel alternative agent for the treatment of human OS.
This work was supported by Youth Foundation of Wuhan Municipal Health Commission (grant no.WX20Q15).
All items in the Submission Checklist have been provided and checked carefully. We declare no conflict of interest, and do not want color reproduction of figures in printed version but want color reproduction in wed version.
[Crossref][Google scholar][PubMed].
Citation: Zhu J, Yan L, Hu R, Li S, Yang C, AN Y (2022) Saikosaponins: A Induces Growth Inhibition and Apoptosis in Human Osteosarcoma MG- 63 Cells via Inactivation of PI3K/AKT Signaling Pathway. Chemo Open Access. 10:164.
Received: 02-Sep-2022, Manuscript No. CMT-22-19549; Editor assigned: 05-Sep-2022, Pre QC No. CMT-22-19549 (PQ); Reviewed: 21-Sep-2022, QC No. CMT-22-19549; Revised: 27-Sep-2022, Manuscript No. CMT-22-19549 (R); Published: 05-Oct-2022 , DOI: 10.35248/2167-7700.22.10.164
Copyright: © 2022 Zhu J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.