Advanced Techniques in Biology & Medicine
Open Access
ISSN: 2379-1764
ISSN: 2379-1764
Research Article - (2022)Volume 10, Issue 5
Malaria, which is caused by Plasmodium parasites, is one of the most important infectious diseases worldwide. Plasmodium parasites in humans use various pathways to communicate within their own population and to manipulate their outside environments, with the ultimate goal of balancing the rate of growth and transmission. For example, circulating extracellular vesicles are increasingly recognized as the key mediators of physiological and pathological processes of a pathogen. Extracellular vesicles are pathogen products consisting of bi-lipid membrane spheres that are secreted from infected-host cells and contain proteins, lipids, and nucleic acids. Based on size and biogenesis, EVs can be categorized into exosomes (released from multivesicular bodies), microvesicles/microparticles and apoptotic bodies (originated by plasma membrane budding). While the function of excretory vesicles have mostly been described in mouse models and some in clinical patients, and some of these are already in place for routine patient care, it is in its early stage in the field of malaria. Given that host-derived EVs can play key role in sensitization of host defense and as of the perspectives of recent studies providing that vesicles are elevated during infection progression and have induced pro-inflammatory activity, they are highly likely to be used as a candidate in producing vaccines against pathogenic diseases affecting millions of human life, as in the case of malaria. This review aimed to discuss the role of excretory vesicles derived from Plasmodium infected red blood cells in the pathogenesis of malaria.
Plasmodium; Red blood cells; Excretory vesicles; Pathogenesis
ABCA1: ATP Binding Cassette Transporter A1; CD: Cluster of Differentiation; EVs: Extracellular Vesicles; IL: Interleukin; iRBCs: infected Red Blood Cells; MPs: Microparticles; MVBs: Multivesicular Bodies; MVs: Microvesicles.
Malaria is a parasitic disease caused by unicellular protozoan of the genus Plasmodium and transmitted by the bite of infected female anopheline mosquitoes. From several species of Plasmodium: Plasmodium falciparum, P. vivax, P. ovale, P. malarae and P. knowlesi are the only parasites known to infect humans [1,2]. Malaria is one of the most important infectious diseases globally. Although malaria is preventable and curable, it still causes significant morbidity and mortality. While examining the global trend of the disease, the African region is home for the highest number of malaria morbidity and mortality. According to the WHO report of 2017 alone, Africa was burdened with 92% of the total cases and 93% of the total deaths worldwide [1].
The pathway of Plasmodium infection is intriguing since its initial entry into the human body to establish its niche in the liver up until its next transmission to the vector. Once injected into humans, only sporozoites that cross the hostile microenvironment and reach to the liver establish its development. At the liver, the parasite is capable of modulating inflammatory response of kupffer cells and hepatocytes [3]. And immune response to liver-stage malaria is limited and slightly mediates type I Interferons (type I IFN) [4]. Whereas several other studies revealed that blood-stage Plasmodium infection has different immune escape strategies enduring long period parasite survival along with development of clinical symptoms and severe disease [5-7].
In the late stage of malaria infection typically due to P. falciparum, infected red blood cells radically change and acquire the ability to adhere to vascular endothelium. The adhesiveness behavior of infected Red Blood Cells (iRBCs) is due to surface expression of antigenically variant parasite proteins. The biology of expression of these proteins on the surface of RBC membrane is considerably interesting, and it plays a role in the cytoadherence and sequestration of the parasite to worsen the disease [8].
It seems that there is a need to explore deeper about the biology and mechanism of pathogenesis of Plasmodium parasites to better understand its success scenario to coexist this long period with humans since its first recognition in 1899. The parasite asexual stage relies on the ability to communicate with one another and with their host, but the mechanisms underpinning this communication are still coming to light. Research in this area has largely focused on the vesicles secreted from iRBCs, many of which down-modulate the host immune response [9,10].
The iRBC provide both host and parasite origin Extracellular Vesicles (EVs), which prominently play in the parasite-and-host interplay in order to regulate host immune responses to the parasite and provide sensing mechanisms for the parasite population. They also transfer information horizontally between cells without direct cellular contact and are involved in a wide range of biological processes such as pathogenesis [11].
There is increasing evidence from in vivo studies demonstrating that specific EVs are released from plasmodium infected human cells. These vesicles play a wide range of roles including physiological and pathological function during malaria infection. It has been also revealed that EVs from plasmodium infected red blood cells contribute to severity of the disease. And that this seminar is providing a brief understanding of the role of secretory vesicles derived from plasmodium infected red blood cells in the pathogenesis of malaria.
Extracellular Vesicles (EVs) consist of bi-lipid membrane spheres that secrete from cells and contain proteins, lipids, and nucleic acids including messenger RNA and microRNA. These molecules are believed to be functional and can transduce signals in recipient cells. For example, EVs from mast cells contain mRNAs that can be transcribed into proteins in the recipient cells [12]. When EVs are taken by other cells, they act as essential mediators of cell-to-cell communication. Even though vesicles are difficult to differentiate; they can be identified based on their sizes, biogenesis and cellular compartment of origin. Further, they can be categorized into exosomes (released from Multivesicular Bodies (MVBs)), Microvesicles (MVs)/Microparticles (MPs), and apoptotic bodies (originated by plasma membrane budding) [13,14].
Exosomes
Exosomes are the smallest average size 40 to 120 nm membrane vesicles which are identified during the study of transferrin receptor trafficking in reticulocytes. Exosomes are released by membrane invagination into the endosome during the production of MVBs. Transferrin receptor mediates intracellular iron uptake which is required for hemoglobin synthesis. Mature reticulocytes contain large sacs filled with small membrane-enclosed vesicles of nearly uniform size within their cytoplasm which can fuse with the plasma membrane and release their internal as exosomes into the extracellular compartment [15,16].
Studies in exosome formation showed that syndecan and syntenin play an underlying mechanism in protein targeting to exosomes sorting and binding. Syntenin is a soluble protein recruited to the plasma membrane by binding to the cytosolic domain of syndecans, which are cell-surface transmembrane co-receptors for adhesion molecules and growth factors. Syntenin interacts through specific motifs in its unstructured N-terminus directly with the ESCRT component Alix and Rab proteins which regulate maturation and fusion of MVBs with the plasma membrane [17].
The endosomal sorting complex required for transport (ESCRT I, II and III) organizes the sorting machinery that delivers different particles (cargoes) from the endosomal surface into its internal and finally into the vacuole lumen. Then in the vacuole, cell-surface cargoes are degraded, endocytosed, and trafficked to endosomes. The ESCRT machinery recognizes their ubiquitin moiety and mediates their different particles before packaging them into Intraluminal Vesicles (ILVs) that bud into the inside of late endosomes, creating MVBs (Figure 1) [18].
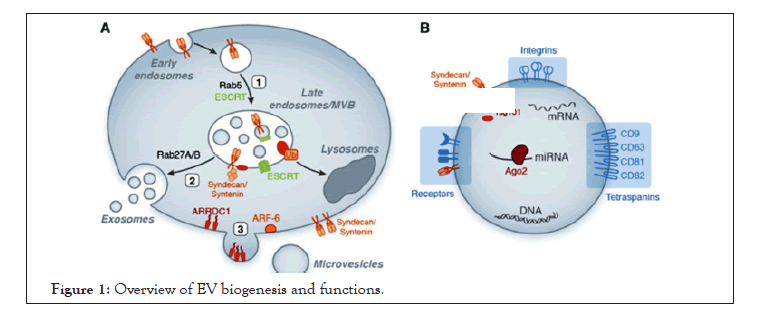
Figure 1: Overview of EV biogenesis and functions.
A. Exosome and MV biogenesis. Exosome generation is initiated through inward budding of early endosomes leading to MVB formation [1] and released when MVBs fuse with the outer cell membrane to release their cargo. ESCRT proteins, in conjunction with additional factors such as syntenin and syndecans, mediate the biogenesis of MVBs and the sorting of specific cargo to MVBs. Rab proteins regulate maturation and fusion of MVBs with the plasma membrane [2]. Microvesicles formation also requires specific factors such as ARF-6, Vacuolar Protein Sorting-associated protein 4 (VPS4), and the plasma membrane protein ARRDC1 which regulates the protein cargo and release of extracellular vesicles [3].
B. EV function. EVs express specific receptors on their surface reflecting their cellular origin (i.e, tetraspanins, and integrins) and contain various bioactive molecules including protein, lipid, DNA, and RNA (including miRNA bound to Argonaute-2 (Ago2)).
Initially, exosomes are important in reticulocyte cargo-disposal for maturation to erythrocytes. They are now recognized to be found in many different types of cells, including dendritic cells, macrophages, B cells, and tumor cells. Exosomes have a role in antigen presentation, immune modulation and the delivery of biologically active molecules for the induction of phenotypic changes. Exosomes released from plasmodium infected RBC found to contain parasite material and pro-inflammatory molecules, and help to induce gametocytogenesis, mediate cell-cell communication between iRBCs, and T cell induction (Figure 2) [13,19,20].
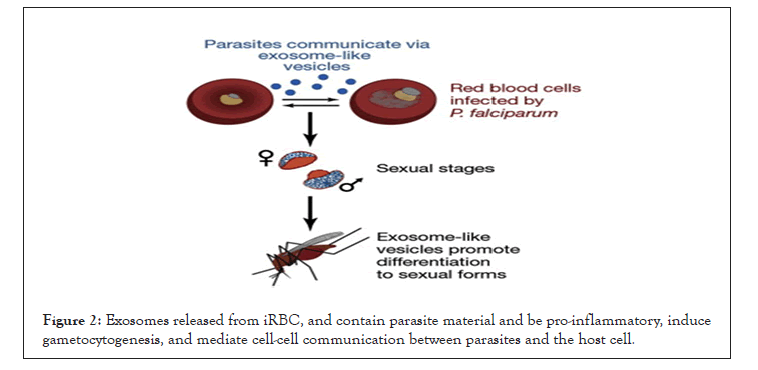
Figure 2: Exosomes released from iRBC, and contain parasite material and be pro-inflammatory, induce gametocytogenesis, and mediate cell-cell communication between parasites and the host cell.
Microvesicles (MVs)
Microvesicles have average size of 50-1000 nm, and formed by dynamic interplay between cytoskeletal protein contraction and phospholipid redistribution with the action of enzyme “flippase” and “floppase” which balances uneven distribution of phospholipid in the membrane from the outer leaflet to the inner translocation and vice-versa, respectively. The enzyme action also activates specific factors such as ADP-Ribosylation Factor 6 (ARF6) required to MVs formation and the plasma membrane protein (Arrestin‐Domain Containing Protein 1) (ARRDC1)) which regulates the protein cargo and release of MVs by outward budding from the plasma membrane [13,21,22].
MVs are submicron plasma membrane fragments shed from the surface of many different cell types including red blood cells during both homeostatic and pathological conditions. For example, the level of MVs has been revealed to be increased during the course of malaria progression and is identified to be involved in cerebral malaria. This is marked with cessation of progression of cerebral malaria upon blockade of production of EVs both in vivo and in vitro models. These vesicles released from iRBC are found to contain parasite material and pro-inflammatory molecules, and function to induce gametocytogenesis, and mediate cell-to-cell communication between parasites [13,19].
Microparticles (MPs)
Microparticles are 100-400 nm sizes and released by the vesiculation of plasma membranes. They also serve in cell-to-cell communication and protein transportation in Plasmodium infected red blood cells. MPs should not be confused with exosomes as MPs originate by budding or shedding from the plasma membrane as opposed to the fusion of the MVBs with the plasma membrane [23].
Apoptotic bodies
Apoptotic bodies are the largest vesicles (500-2000 nm), which are released during outward blebbing from the plasma membrane of cells undergoing apoptosis and carry whole organelles on their inside. When cells undergo apoptosis, the ATP Binding Cassette Transporter A1 (ABCA1), which promotes membrane microvesiculation, migrates to the outer leaflet of the lipid bilayer membrane. Apoptosis is characterized by a series of dramatic alarms to the cellular manner that contribute not only to cell death, but also prepare cells for removal by phagocytes and prevent unwanted immune responses. The destruction phase of apoptosis is coordinated by members of the cysteine proteases. These proteases target many hundred proteins for restricted proteolysis in a controlled manner that minimizes damage and disruption to neighboring cells [13,24].
Mosquitoes during dermal blood feeding on the host inject sporozoites, which then migrate to the liver where they invade hepatocytes. Inside the hepatocyte, sporozoite develops into several thousand merozoites, which are released into the bloodstream and invade RBCs. During the blood-stage of the parasite life-cycle, ring stage parasites develop into replicative schizont forms that release multiple invasive daughter merozoites. The apical end of the merozoite is demarcated by the polar rings which are membrane-bound organelles: Rhoptries (two prominent pear-shaped), micronemes (ovoid bodies), and dense granules (spheroid vesicles) are located at the anterior end of the merozoite. The contents of these organelles play a role in the binding and entry of the merozoite into the host cells [25].
During the process of malaria infection, iRBCs release different products into the host circulation which then to be recognized by toll like receptors (TLRs) and scavenger receptors (such as CD36), and up taken by other iRBCs. Of the products, excretory vesicles originating from iRBCs contain parasite origin small regulatory RNAs and host origin miRNAs help in cellular communication and regulation of endothelial cell barrier [26]. Other than host-directed function of the vesicles, they can also trigger differentiation of the parasite into gametocytes, and provide increased survival mechanisms of the parasite during stress in other iRBCs [27,28]. On the one hand, these products are going to be recognized by host innate immunity and T-cells to induce pro-inflammatory immune responses to control the status of malaria infection. This process also helps to regulate production of pro-inflammatory cytokines, including Interleukin-6 (IL-6), Tumor Necrosis Factor (TNF) and Interferon-γ (IFN-γ) in the course of malaria progression. These cytokines are considered to be involved directly in the progression of severe malaria such as severe anemia, cerebral malaria and organ damage [29].
Infection by Plasmodium parasite can profoundly modify the composition and function of EVs in immune cells [30]. Several pieces of evidence showed that EVs in Plasmodium infected individuals contribute to malaria-associated clinical symptoms, particularly severe forms of the disease. It has been shown that naïve macrophages exposed to EVs derived from malaria-exposed macrophages produce pro-inflammatory cytokines whereas vesicles from non-exposed macrophages are non-stimulatory [31].
Based on the status of immunity of the host, EVs can induce adaptive responses or inhibit inflammation. The inflammatory response is commonly caused by the recruitment and activation of leukocytes which are often associated with the increased secretion of inflammatory cytokines: IL-1β, IL-4, IL-6, IL-8, IL-10, IL-12, TNF and IFNγ. However, the role of parasite derived vesicles is detrimental for this scene. The activation of immune cells also requires interactions with Clusters of Differentiation (CD) proteins and the stimulation of CD proteins can act as a marker for pro-inflammatory response. These factors are part of a T Helper 1 (TH1) cell response that results in the activation and recruitment of macrophages, monocytes and other leukocytes to the site of infection (Figure 3) [32,33]. Whereabouts modification of parasite genetics which directly affects the content of vesicles released from iRBCs consecutively alters the interplay with host immune response. For example, EVs derived from iRBCs with PfEMP1 knockout plasmodium parasite induced altered pathways involved in host defense response, stress response, and cytokine production [34].
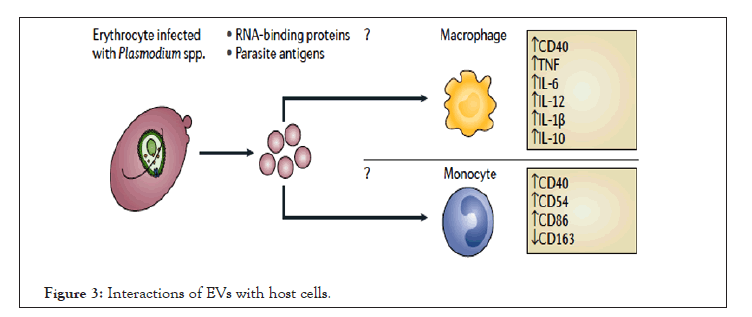
Figure 3: Interactions of EVs with host cells.
EVs contain parasite protein and RNA which elicit a pro-inflammatory response, which increases the parasite burden in the host and/or disease. Upward arrows denote an increase in expression or secretion and downward arrows denote a decrease in expression or secretion.
A study had shown that naive monocytes were activated by EVs derived from iRBCs in vitro with P. falciparum. Activated monocytes were then shown an upregulation of inflammatory response markers consisting CD40, CD54 and CD86, and a downregulation of CD163. These EVs also activated macrophages and stimulated the production of IL-10 and pro-inflammatory cytokines, specifically IL-6, IL-12 and IL-1β [30]. On the other hand, EVs released from iRBCs also deliver immunogenic Variant Surface Glycoprotein (VSG) onto the surface of erythrocytes [35].
Similarly, vesicles from malaria-infected mice induce potent activation of macrophages in vitro as measured by CD40 up-regulation and TNF production. These vesicles had significantly induced activation of macrophages than from intact infected red blood cells. However, activation of macrophages only by vesicles is completely abolished in the absence of MyD88 and TLR-4 signaling [36]. Interestingly, P. falciparum infection derived vesicles are rapidly internalized by macrophages and trigger strong pro and anti-inflammatory cytokine responses (Figure 4) [37].
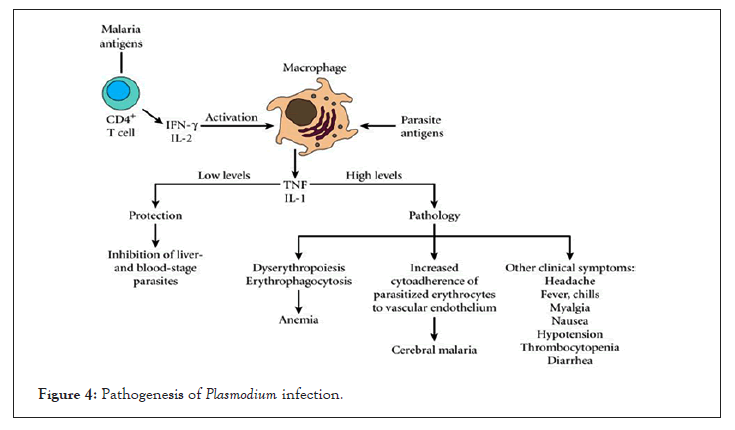
Figure 4: Pathogenesis of Plasmodium infection.
On the one hand, comparison of level of EVs secreted from iRBC with either P. vivax, P. malariae or P. falciparum demonstrated that the highest level was from patients with severe malaria due to P. falciparum [31]. The production of vesicles was increased with disease progression, and that the host pro-inflammatory molecules may not be required for the generation of these vesicles. However, these vesicles can help to transfer messages such as examining receptors and signaling pathways required for macrophage activation [23,38]. Likewise, acute P. vivax malaria is related to pro-inflammatory responses and infection derived vesicles may play important during acute infection in non-immune patients [38].
Others have demonstrated that the level of endothelial-derived EVs is high in patients with severe cerebral malaria suggesting that the EVs are associated with malaria cerebral pathogenesis, endothelial cell inflammation and local cell sequestration. As such mice with ABCA1 knock-out behaving reduced ability to produce MVs were protected from cerebral malaria [39]. On the other hand, mouse in vitro models suggest that EVs derived from platelets and endothelial cells contribute to the overall inflammatory condition [40]. Most importantly TNF and TGFβ1 are predicted to regulate cerebral malaria associated proteins in MVs. Overall, these findings are coherent with the association of MVs with cerebral malaria and the involvement of TNF with it [13].
Likewise, it has been illustrated that the level of vesicles originating from platelets, erythrocytes, and endothelial cells was high during cerebral and non-cerebral severe malaria compared to uncomplicated malaria. Interestingly, vesicles originating from platelets can bind to P. falciparum iRBC in a PfEMP1-dependent manner, and induce iRBC cytoadherence to endothelial cells. This systematic contribution of vesicles in cerebral malaria revealed that vesicles encourage cerebral pathology by stimulating iRBC cytoadhesion in the brain [11].
Whereas other studies have demonstrated several proteins that are carried across the parasite membrane and the surrounding parasitophorous vacuole membrane into the host cell [41]. Protein trafficking to the membrane surface of iRBC is via parasite-induced membrane platforms that are anchored to the host cell cytoskeleton essential for vesicles release and uptake by recipient cells. Intriguingly, the lack of trafficking of parasite origin vesicles across infected RBCs in sickle cell hemoglobin mutation may contribute protection of severe malaria [42].
During high release of EVs in plasmodium infection, they induce strong pro-inflammatory effects, stimulating macrophage CD40 surface expression and TNF release in a dose-specific manner. TNF release and CD40 up-regulation as a measure of macrophage activation, and it is likely that other pathways (including other TLRs) are also activated by MVs and induce a release of cytokines. Monocyte-derived macrophages are stimulated with MVs, they up-regulated the expression of IL-1β, IL-6, IL-10 and IL-12, and specifically released IL-10 and TNF, showing that vesicles have an effect on host cells [30].
The development of an effective vaccine against malaria is one of the major global interests, but due to the complexity of the Plasmodium parasite life cycle, and highly variable surface protein redundancy it still remains a challenge. Although many candidate antigens have been evaluated as potential antimalarial vaccines, it is hard to name a single vaccine in routine clinical use [43].
An effective immune response must act quickly in order to inhibit Plasmodium sporozoites in their minutes-long journey from the skin to the liver. The immune system should provide both humoral and T-cell responses that can prevent hepatocyte invasion. RTS, S is the leading pre-erythrocytic malaria vaccine and showed phase III clinical trial of a malaria vaccine, efficacy against clinical malaria in children during the 18 months following dose 3 was 46% overall. Generally, other pre-erythrocytic vaccine strategies in development include the multiple epitopes Thrombospondin-Related Adhesion Protein (TRAP) and whole-organism sporozoites strategies are on the way. However, several efforts are still underway to develop an effective and long-lasting antimalarial vaccine. There is considerable interest in utilizing EVs to enhance vaccine delivery and mass production of EVs, such as exosome mimetic nanovesicles, could provide a viable therapeutic approach to antimalarial vaccine development [44-46].
Studies which used iRBC-derived EVs to successfully immunize mice against lethal infection showed the immunization potential of parasite derived exosomes [47]. Although, most of the anti-malaria vaccine researches are focused on the use of synthetic MPs/microspheres, such as Poly Lactic-co-Glycolic Acid (PLGA), with parasite antigens including Merozoite Surface Proteins (MSP1,MSP2,and MSP3), serine-repeat antigen, erythrocyte-binding antigen, Ring-infected Erythrocyte Surface Antigen (RESA), Glutamate-Rich Protein (GLURP), and Apical Membrane Antigen-1 (AMA1), they showed improved humoral and cell-mediated immune responses compared to standard adjuvant vaccination, highlighting PLGA vesicles as an improved immunization strategy [48,49]. Others are developing anti-malaria vaccines using PLGA to deliver Plasmodium antigen-encoding plasmid DNA to increase vaccine immunogenicity to elicit wide-range of immune responses [50].
Due to the recent interest in halting malaria transmission, the intention of developing malaria transmission-blocking vaccines is advanced. And a successful transmission-blocking vaccine would need to induce neutralizing antibody against the gametocyte and/or ookinete sexual stages thereby blocking fertilization in the vector. Some of the lights of transmission-blocking vaccines underway include ookinete surface proteins P25 and P28 of P.falciparum and P.vivax. However, due to the lack of natural antigen presentation of red blood cells, this strategy lacks natural boosting and can result in limited efficacy. But the use of biodegradable packages containing EVs as an antigen could progressively elicit long-lasting functional antibody responses to make an effective vaccine [51]. Further manipulation of EVs with improved adjuvants can trigger greater humoral immunity and potency of vaccination strategy. Likewise, a combination vaccine containing leading candidate antigens, delivered via EVs or mimetic nanovesicles, could be the best strategy for vaccination against malaria [52,53].
Plasmodium parasites and infected host cells use Extracellular Vesicles (EVs) for intercellular communication and host manipulation. By using various mechanisms to generate EVs and transferring a wide range of molecules through EVs, plasmodium parasites are able to establish an infective niche, modulate the host immune system and cause disease. Besides impact on the host, EVs are able to transfer virulence factors, drug-resistance genes and differentiation factors among the parasite population.
It should be noted that EVs could have originated from either normal host cells or infected cells, and both contribute to malaria pathogenesis. Those host-derived EVs have been implicated in induction of cytoadhesion of iRBC, and microvascular sequestration in the brain whereas parasite materials help to induce pro-inflammatory responses, transfer functional microRNA, and mediate cell-cell communication among parasites. Despite EVs function in malaria pathogenesis, one of the positive scientific impressions in the use of plasmodium EVs can be their role to induce antibody mediated immune protection as demonstrated in mouse models to generate effective anti-malaria vaccines.
However, there could be several challenges in the study of EVs to apply them in medicinal practice for public clinical practice. Some of the challenges include high heterogeneity of human samples, and discrepancies in the methodology used to isolate EVs where all likely result in varying compositions of the materials being investigated, and causes varying results. Therefore, a consensus on sample preparation is required to ensure that the same vesicle types are being investigated between groups. In addition, it remains difficult to provide concrete evidence whether malaria-associated inflammation causes increased production of EVs or EVs themselves are the key mediators of malaria pathogenesis. Further investigation, in particular more detailed proteomic and genetic analyses of the content of EVs during malaria infection, is still required to tease out effect-and-cause analysis to correlate EVs and malaria pathogenesis.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Minwuyelet A, Abiye M (2022) Role of Secretory Vesicles Derived from Plasmodium Infected Red Blood Cells to the Pathogenesis of Malaria: A Review. Adv Tech Biol Med. 10:360.
Received: 06-Jun-2022, Manuscript No. ATBM-22-17777; Editor assigned: 09-Jun-2022, Pre QC No. ATBM-22-17777 (PQ); Reviewed: 23-Jun-2022, QC No. ATBM-22-17777; Revised: 30-Jun-2022, Manuscript No. ATBM-22-17777 (R); Published: 07-Jul-2022 , DOI: 10.35248/2379-1764.22.10.360
Copyright: © 2022 Minwuyelet A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.