Journal of Chemical Engineering & Process Technology
Open Access
ISSN: 2157-7048
ISSN: 2157-7048
Research Article - (2025)Volume 16, Issue 3
Now a day science focuses towards sustainability and green technology. Chitosan is an biodegradable and renewable green material with diverse functionalities. It is most promising materials used prolifically in numerous applications, such as catalysis, adsorption, delivery of therapeutic agents, and remediation. In this review attempts to collect useful information with respect to the use of chitosan and modified chitosan preparations loaded with metal ions, complexes and metal particles as catalysts in organic reactions and transformations. These include coupling reactions, oxidations, hydrogenations, reductions, addition, and other organic reaction. Because chitosan molecules having ability to coordination with metal and form a metal-chitosan complex due to presence of large amounts of NH2 and OH functional groups available in chitosan structure for chemical modification reaction. In recent years chitosan widely used in Cu, Pt, Ru, Au, Rh, and Pd and metals support catalyst, and showing excellent catalytic properties.
Chitosan: Catalyst: Metals: Organic reaction
Chitosan proven as a promising catalyst due to its good coordination ability to the metal compounds, chitosan and its derivatives can be used as a kind of good catalysts carrier. Recently, the catalytic properties of chitosan and its derivatives supported metal catalyst have been attracted more attentions. Based on the kind of reactions, the applications of chitosan and its derivatives supported metal catalysts in the catalytic asymmetric reaction, allylic substitution, coupling, addition, oxidation, reduction and some other reactions are reviewed.
Chitosan contains large amounts of NH2 and OH functional groups available for chemical modification reaction (Such as the preparation of chitosan derivatives and complex metal ions). In recent years, chitosan as a biopolymer widely used in Cu, Pt, Ru, Au, Rh or Pd and other precious metals supported catalyst which supported metal catalysts have been widely used in oxidation, reduction, addition and other reactions, showing excellent catalytic Can. Guibal, et al. [1]. Early chitosan and its study on morphology and structure of derivative supported metal catalysts and catalytic reaction has been reviewed in detail. Currently, chitosan and its derivatives are catalyzed the reaction is mainly used as a carrier of the catalyst, but the amino group in the molecule can be used as organic bases, it has also been reported as a catalyst in recent reports reactions. In this dissertations, the type of reaction the main line, in recent years chitosan and its derivatives supported metal catalysts for catalytic organic reactions were review (Figure 1).
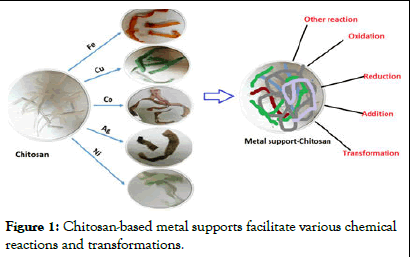
Figure 1: Chitosan-based metal supports facilitate various chemical reactions and transformations.
Asymmetric reactions
Chitosan has a natural chiral character and can be used in asymmetric catalysis. Which play an important role in the study of chitosan and its derivatives supported metal catalyst. Asymmetric synthesis of, build an efficient catalytic system analogous enzymes, with There's important meaning. Gagnon et al., using modified chitosan and cymene dichloride ruthenium catalyst system, a catalyst was prepared using chitosan as a ligand, for the asymmetric catalytic reduction of acetophenones with methanol/ isopropanol as solvent and an alkali sodium isopropoxide, yield phenyl ethanol 81%, and enantiomeric excesses up to 72%, with the intrinsic chirality of chitosan being the only source of enantioselection. Although this reaction is an enantioselective (Figure 2).
Figure 2: Catalyst facilitates the conversion of phenol to its derivatives.
Ricci et al., synthesized aerogel chitosan, can be used in the aqueous phase asymmetric aldol condensation. It catalyzes pnitrobenzaldehyde and ring cyclohexanone condensation reaction, the condensation product isolated yield of the product and the anti-configuration when the reaction system was added (Figure 3).
Figure 3: Crab-inspired catalyst enhances selective reactions with high enantioselectivity.
2,4-dinitrophenol or stearic acid, the yield can be significantly increased After four cycles, the agent still maintains a high catalytic activity (yield 87%) and (anti-configuration product of enantioselectivity 83%) (Figure 4).
Figure 4: Chitosan catalyzes reaction with high yield and enantioselectivity.
Cui et al., first discovered chitosan load L-proline catalyst may various aromatic Asymmetric Henry reaction of aldehyde with nitromethane, water can swell chitosan load L-proline soluble micelles are formed, as a heterogeneous catalyst, the sol-gel system has high cycle performance.
Crucianelli et al., to prepare a series of modified chitin, chitosan methyl rhenium trioxide catalyst load. In the catalytic epoxidation of olefins, catalyst was found to have a good catalytic activity, the catalyst is loaded onto two silicon oxide, the catalytic activity is further improved catalytic rans β-. A epoxidized styrene-based conversion of the highest 98%, ee of the product was 98% [2-5].
Allylic substitution reactions
Transition metal-catalyzed allylic substitution reactions are found in organic synthesis Important academic significance. The catalytic reaction has versatility, mild conditions, regioselectivity and stereoselectivity changing characteristics. Dez, et al. Preparation of chitosan sugar-loaded ionic liquids catalyze Tsuji-Trost Ally with Pd complex substitution reactions [6,7] (Equation 2). Catalysts have the advantage of their wide applicability The amount of catalyst is small, easy to separate. When Trisulfonated Triphenylphosphine (TPPTS) as a palladium ion ligand, the number of cycles can be up to 10 times or more.
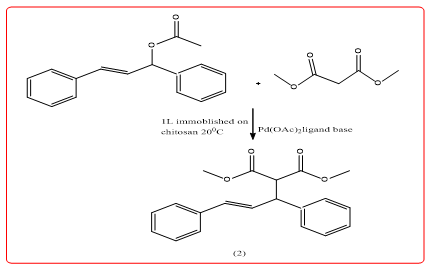
N-Allylation of amines with allyl acetates using chitosanimmobilized palladium a simple procedure for N-allylation of amines with allyl acetates has been developed using a biodegradable and easily recyclable heterogeneous chitosansupported palladium catalyst. The general methodology, applicable to a wide range of substrates, has sustainable features that include a ligand-free reaction with simple workup, recycling and reusability of the catalyst (Figure 5).
Figure 5: Chitosan-supported Pd catalyst enables efficient CN coupling reaction overnight.
The coupling reaction
Coupling reaction of carbon-carbon bond formation is one of the important chemical reactions, These reactions can be used to convert simple reaction precursors to complex structures molecules. Metal complexes of chitosan and its derivatives as the coupling reaction applied is relatively broad, including Heck, Ullmann, Suzuki and Sonogashira and other reactions. Calo, et al. used chitosan supported Pd catalyst halobenzene and Heck reaction of butyl acrylate [8,9] (Equation 3) with Tetrabutylammonium Bromide (TBAB) as solvent and Tetrabutyl Ammonium Acetate (TBAA) as base to catalyze bromobenzene and n-butyl acrylate coupling reaction, due to the formation of highly active Pd colloid stable in the reaction solvent, under the action of alkali can quickly shape into Pd-H active intermediates, the reaction can be achieved within 15 min 99% conversion rate.
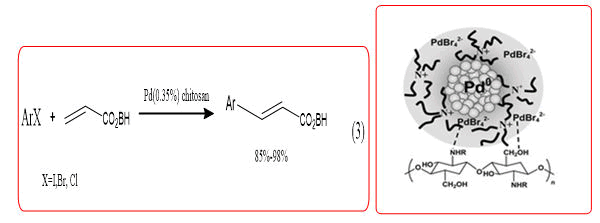
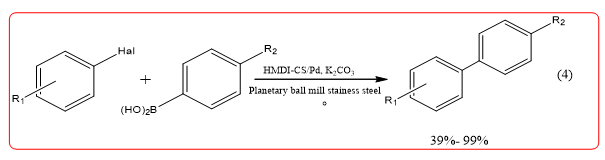
Chitosan, Pd (OAc) 2, 4,4-bicyclic Hexamethylene Diisocyanate (HMDI) blend prepared HMDI cross-linked shell glycan loaded Pd catalyst, this catalyst can be used for halogenated aromatic Suzuki Casing coupling reaction (Equation4), and to obtain better yields. Lee, et al. preparation of polysaccharide-s-triazinemethyl polyethylene glycol and methyl polyethylene glycol Pd catalyst, in the absence of a phase transfer reagent, Suzuki reverse can be efficiently catalyzed should, when using CS-gmPEG350Pd (0) as a catalyst, the amount of Pd is 0.5 mol%, biphenyl yield of 92% [10].
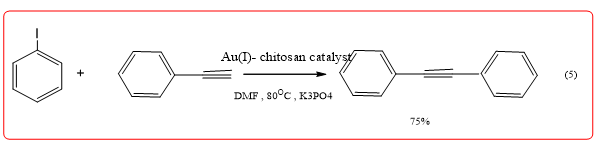
Quignard, et al. was dried by supercritical CO2 preparation of chitosan fiber dimensionally charged nano Au catalyzes Sonogashira reaction (Equation 5) and aryl boronic acids of the self-coupling reaction, by XPS analysis, the catalyst gold loading was 0.5 wt% (Au0 content of 70.3%, Au3+ was 16.4%, Au+ is 13.2%), where Au+ and Au+3 Sonogashira reaction and catalyze aryl basic boronic acid self-coupling reaction of active species by controlling the gold nanoparticles in the shell poly sugar surface growth, enabling it to form a catalytically active species from While efficiently catalyzing the Sonogashira reaction [11,12].
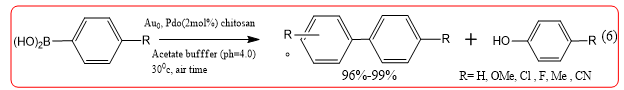
Aerobic oxidative homocoupling of arylboronic acid under acidic aqueous conditions (pH 4.0) using bimetallic Au/Pd alloy nanoclusters stabilized by chitosan has been investigated. It was found that a Au0 .81Pd0.19 catalyst (3.1 ± 0.8 nm) exhibited superior catalytic activities as compared to monometallic Au (2.3± 0.3 nm) and other series of bimetallic nanoclusters, giving the corresponding biaryls in nearly quantitative yield (Equation 6).
Alesi, et al. used chitosan supported Pd catalyst, the microwave strip. Under the catalytic oligothiophene coupling reaction. It is an innovative heterogeneous procedure for the preparation of highly pure thiophene oligomers via microwave-assisted Pd catalysis by using silica and chitosan-supported Pd complexes. This approach is very efficient and greener than the existing homogeneous methodology as it combines a very efficient reaction with improved catalyst separation. Our new, efficient and cleaner microwave approach smoothly afforded the preparation of coupled products in high yields (up to 87% isolated yield, 30–100 min). Iodthiophene or activated bromides were employed as starting materials and KF as base. The microwave reaction was carried out in aqueous ethanol. The heterogeneous catalyst can be easily removed from the reaction mixture by filtration and reused in consecutive reactions (up to 4 times the reaction than the traditional homogeneous catalysis (Figure 6).
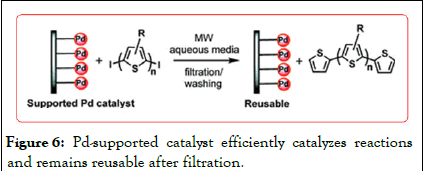
Figure 6: Pd-supported catalyst efficiently catalyzes reactions and remains reusable after filtration.
Addition reactions
The chitosan complex was prepared Au supported silica The catalyst was used for the selective hydrogenation of alkynes octyne as a substrate, at 100°C for 22 h, the substrate conversion rate 100%, ketone and imine selectivity of 36% and 64% respectively Cheng, the catalyst is not dissolved, can fully guarantee the catalyst activity and service life (Figure 7).
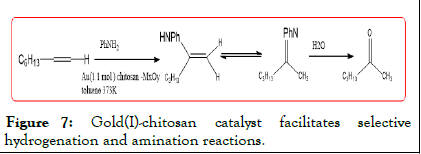
Figure 7: Gold(I)-chitosan catalyst facilitates selective hydrogenation and amination reactions.
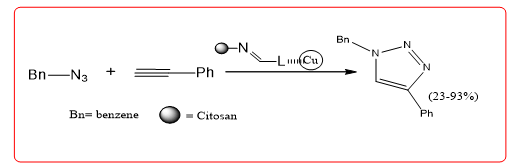
In 2002, Sharpless, et al. reported a Cu (I) catalyzed azide engagement The cycloaddition of the alkyne with the terminal alkyne. The presence of the Cu (I) catalyst renders this class cycloaddition reactions can not only be carried out under mild conditions, but also extremely high the area selectivity and yield are seen as a model of "click chemistry." Taran Schiff base Cu (I) complex and the like a first series of chitosan prepared the catalyst catalyzed the azide cycloaddition reaction (Equation 7), obtaining a comparison good results. When the choice of ligand, the amount of catalyst 0.1 mol%, EtOH and H2O as the solvent, a yield of 99%.
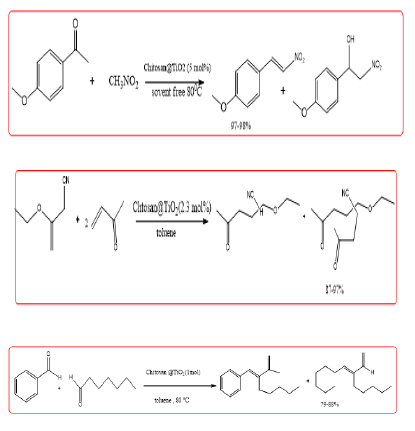
El Kadib, et al. a chitosan hydrogel-supported titania catalyst prepared. And used to catalyze Henry (Figure 9), Michael addition reaction (Figure 10), knoevenagel reaction (Figure 11), the catalyst has good catalysis performance, when to Ti (Acac)2 (O i-Pr)2 precursor synthesized chitosan @TiO2 as a catalyst, yield of 8 to 10 were 97%, 97%, 86% (Figure 8).
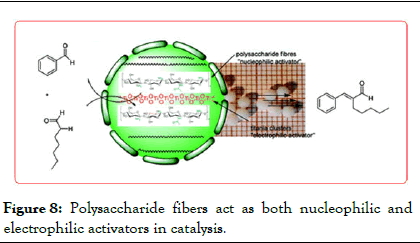
Figure 8: Polysaccharide fibers act as both nucleophilic and electrophilic activators in catalysis.
Shukla, et al. the chitosan simple process to prepare a hydrogel, after drying and crushing was used directly in the catalytic reaction jasmine aldehyde synthesis of 10, the reaction At 160°C without solvent, the conversion of the substrate is greater than 99%, Mo. The yield of aldehydes is up to 88%.
Oxdiation
Cyclohexanone and cyclohexanol are important organic chemical raw materials supported metal complex catalysts catalyze cyclohexane oxidation to produce cyclohexanone and rings The work of hexanol has been reported, the catalyst has been reported chitosan and modified chitosan load Fe, Cu, Co, Ni, Au, Pd complexes, etc. Guo, et al. a chitosan supported porphyrin Fe (III) prepared in the absence of a solvent under the catalytic oxidation of cyclohexane, it was found that the chitosan supported catalyst ratio the catalytic activity of pure porphyrin Fe (III) was higher at 418 K and 0.8 MPa the cyclohexane conversion was 10.48% with cyclohexanone and cyclohexanol selectivity was 79.2%. The conversion frequency was 22 times that of porphyrin alone.
Labhsetwar, et al. chitosan loaded mesoporous TiO2 prepared, ZrO2 , Al2O3 and loaded Cu-Mn catalyst, and toluene for acetaldehyde catalytic oxidation, the catalytic activity of the order of Cu-Mn/TiO2>Cu-Mn/ZrO2> Cu-Mn/Al2O3 . Where in the Cu-Mn/TiO2 and Cu-Mn/ZrO2 In toluene to achieve complete conversion 350°C, the Cu-Mn/Al2O3 in 400°C to achieve complete conversion; when acetaldehyde as a substrate at 80°C,10 h inter cholobenzaldehyde in 97% yields.
Li, et al. preparation off dendritic tin with a chitosan supported by solid phase method catalyst, Baeyer-Villiger oxidation of ketones to rearrangement reaction. This catalyst catalyzes the adamantanone in the presence of hydrogen peroxide BaeyerVilliger oxidation up to 99% conversion rate, selectivity 100%. Since the catalyst is insoluble in organic solvents, it can be recycled several times use (Figures 9 and 10).
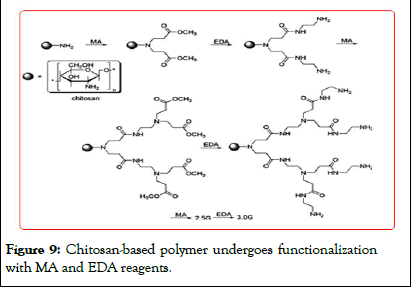
Figure 9: Chitosan-based polymer undergoes functionalization with MA and EDA reagents.
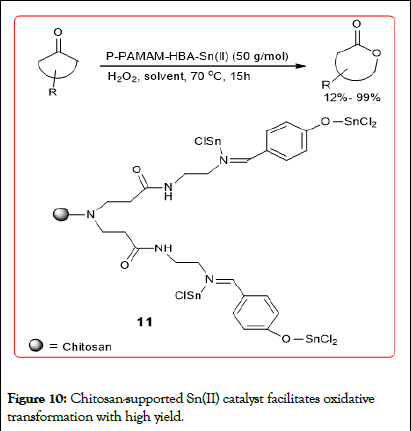
Figure 10: Chitosan-supported Sn(II) catalyst facilitates oxidative transformation with high yield.
Reduction reaction
Gong, et al. SiO2 -CS- schiff base Pd catalyst prepared by catalytic reduction of acetophenone to, PdCl2 , and compared to SiO2 - CS-Pd, CS-Schiff-Pd and SiO2 -CS-Schiff-Pd has a stronger catalytic activity, acetophenone can be directly reduced to ethylbenzene. When ethanol as a solvent, hydrogen When a flow rate of 45 mL/min H2 , a temperature of 313 K, the yield of ethylbenzene may a modified chitosan-supported Pd catalyst to reach 100%. Bajaj, et al. preparation of N Agents, and catalytic reduction phenyloxirane, wherein the N-amino modified chitosan base Pd complex as a catalyst, the yield of 2- phenylethanol was 85%. Ondruschka, et al. a hexamethylene diisocyanate modified chitosan prepared Glycosyl Pd (II) complex and catalyzes cyclohexenedione, chalcone, lemon aldehyde, 1,2-diphenyl acetylene, N-benzyl aniline and other olefins and ethylenically α,β unsaturated. And aldehyde and ketone hydrogenation, its double bond selectivity can reach 99% (Figure 11).
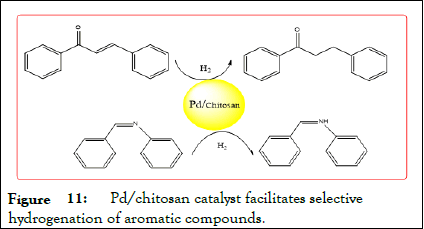
Figure 11: Pd/chitosan catalyst facilitates selective hydrogenation of aromatic compounds.
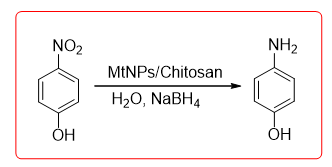
Chitosan load Ag and Au can catalyze the nitrophenol eduction (Equation 13). Nanogold particles stably exist on chitosan carriers, following no evidence of catalyst deactivation was found after 11 cycles of use. Chatto-padhyay, et al. preparing chitosan nano-Ag complex catalyst, this catalyst The chelating agent can be used to catalytically reduce 4-nitrophenol, and the catalyst can be reused after 10 times still maintain a high catalytic activity.
Other reaction
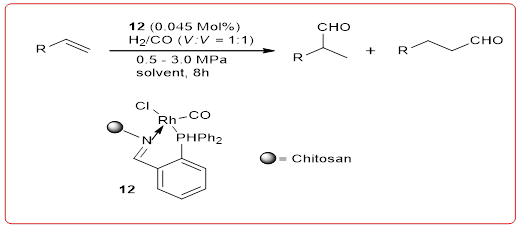
Hydroformylation reaction: Hydroformylation of olefins is an important class of homogeneous catalytic reactions, has a wide range of industrial prospects for pesticides, spices, medicines as well as natural the product is important synthesis. Smith, et al. synthesized chitosan Schiff base sugars Rh (I) complex catalyst 12, catalytic hydrogenation of 1-octene a acylation (Equation 14). Catalytic synthesis of 1-octene aldehydes is best The reaction temperature is 75°C, the pressure of the mixture is 3.0 fMPa, orming 52%~95%. Aldehydes and a portion of isooctyl lean compared to such catalysts [Rh (CO)2-(acac)2] catalysts have similar activity and higher selectivity, in cycle after four cycles of use, the catalytic reaction is still able to achieve 75%~79% turn Rate.
Carbon monoxide and carbon dioxide insertion reaction
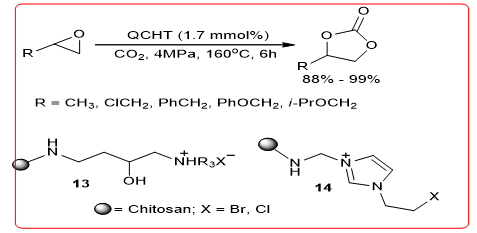
Quaternary ammonium compounds–Park, and He, et al. preparation of chitosan (QCHT) 13 for the synthesis of the carbon dioxide reaction catalyzed epoxy compound and acid ester compounds (Equation 15), conversion rate=when R=Me, X=I 100%, carbonate yield greater than 99%. The catalytic mechanism may be because the OH in chitosan forms hydrogen bonds with the epoxy compound, which promotes the negative ionization of the halogen addition of the sub-β carbon atoms and an epoxy compound, a ring opening formed intermediate, then, further, adding CO2 to form cyclic carbonate lipid compound. Zhang Etc. Chitosan 1-ethyl-2-methyl imidazolium complex was prepared substituting bittern 14, a catalytic reaction of an epoxy compound with carbon dioxide to carbonates synthesis compound, the reaction is carried out in the microwave, the conversion rate of up to 95%.
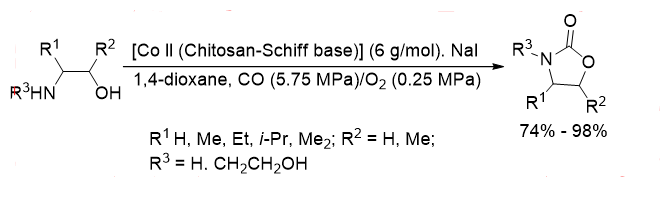
Carbonyl insertion reactions are heavier in organic cemistry and in industrial applications The desired reaction is achieved by the carbonyl insertion of C-X (X=Cl, Br, etc.) can synthesize a series of carbonyl compounds, in line with "atomic economy" and "green requirements color chemistry " Xia, et al. preparation of chitosan chitosan-Schiff Base-Co (II) complexes catalyze 2-amino alcohol under the action of sodium iodide. The carbonylation reaction (Equation 16), and a higher yield is obtained when R 1=R3=H, R 2=Me, the 2-amino alcohol conversion was 100%.
Chitosan is a kind of natural macromolecular compound, which contains a large amount of amino groups and hydroxyl, easy to modify and coordination with metal ions to form functional chitosan derivatives and chitosan supported metal catalysts. Ammonia on chitosan molecules as a base of organic base catalyst research also aroused people's attention, its function modification is expected to become a new generation of organic base catalyst. Chitosan and its derivatives chiral center on chitosan and its supported metal catalysts asymmetric catalytic reaction have an important effect. Chitosan and its derivatives dissolved adjustable solution, with the metal complex formed catalyst has good stability as a low cost, environmentally friendly catalyst and catalyst carrier, chitosan and its functional derivatives in heterogeneous Catalysis Industry the application in the future is also an important direction of research.
The authors declare no competing interest.
Janntun Zia wrote the final draft.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Zia J (2025) Role of Metal Support Chitosan-based Catalyst in Organic Reactions. J Chem Eng Process Technol. 16:542.
Received: 05-Jun-2024, Manuscript No. JCEPT-24-31877; Editor assigned: 10-Jun-2024, Pre QC No. JCEPT-24-31877 (PQ); Reviewed: 24-Jun-2024, QC No. JCEPT-24-31877; Revised: 25-Jul-2025, Manuscript No. JCEPT-24-31877 (R); Published: 02-Aug-2025 , DOI: 10.35248/2157-7048.25.16.542
Copyright: © 2025 Zia J. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.