Journal of Developing Drugs
Open Access
ISSN: 2329-6631
ISSN: 2329-6631
Review Article - (2013) Volume 2, Issue 2
Medications produced as semi-solids type product such as creams, ointments and lotions are based on emulsion or suspension type systems consisting of two or more incompatible materials. In order to be manufactured, these dosage forms need specific flow properties so they can be placed into a container, remain stable over time, dispensed, handled and properly applied to the affected area by patients. Rheology is therefore crucially important as it will directly affect the way a drug is formulated and developed, the quality of the raw and finished product, the drug efficacy, the way a patient adheres to the prescribed drug, and the overall healthcare cost. It can be concluded that there are inherent and independent factors that affect the flow property of a medicated material during every stage of its manufacturing all the way to its use.
<Keywords: Pharmaceutical formulation; Rheology; Viscosity; Suspensions; Rheology modifiers; Hydrophilic polymers
The following Stokes equation is familiar to most; expressing the rate of sedimentation of suspended particles over time in a liquid vehicle:
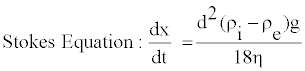
This equation estimates the sedimentation rate based on certain physical characteristics of the suspension. These characteristics include the diameter of suspended particles (d), acceleration due to gravity (g), density of the particles (ρi) and external phase (ρe), and the external vehicle viscosity (η). Some of these variables themselves are also dependent on other factors which can affect the rate of settling. For example, vehicle density and viscosity are both dependent on the temperature of the material. If we look closer at the viscosity variable in this equation we can see some fundamental questions that may arise. For example, will the rate of sedimentation change if we start mixing the suspension? How will sedimentation be affected if we use different tools for the mixing process, for instance hand mixing, high shear blender, or a heavy duty mixer? Assuming particle size is not affected by the way a suspension is mixed, the equation leaves only one factor that can be affected, viscosity. However, if the vehicle is water, this assumption does not hold true. Water has a constant viscosity and is always the same no matter how vigorous it’s agitated or mixed as do all Newtonian fluids. Nevertheless, we do know that the viscosity of a water-based vehicle is affected by how much solid particles are added into it; typically increasing in viscosity with higher solids content.
Certain properties of the particles making up a suspension can also affect viscosity. Particles that are porous will have internal space to accommodate the liquid vehicle, by which the viscosity of the whole suspension will change. Another fundamental question that may be asked is how the viscosity will change if the suspension contains particles that deviating from a spherical shape? Studying the micromeritics of particles is already known to be important to pharmaceutical scientists when characterizing certain properties and behaviors particles possess. Since we know particle shape has an effect on flow properties in the solid state, we may expect similar behavior when evaluating particle behavior in mixed phase suspension systems. The study of rheology can be used to evaluate the behavior of particles in a liquid vehicle. When stress is applied to spherical particles in a liquid, the applied stress is dampened as the particles easily slide over one another to maintain a steady viscosity, or Newtonian behavior. If stress is applied at a faster rate, the spherical particles slide faster over each other to maintain their history of viscosity. For spherical particles, stress applied at a faster rate does not have a significant effect on viscosity due to their simple geometry and minimized surface area. Spherical particles therefore experience minimized frictional forces which facilitate the sliding process and cause the viscosity to be almost insensitive to shear rate. A different behavior may therefore be expected for non-spherical particles (Figure 1). For irregular shaped particles, when stress application is slow (e.g., hand mixing with approximately 20 rpm rate of agitation), one may observe similar behavior as seen with spherical particles at the same solids content. However, if the rate of agitation is increased using a high shear mixer at 2000 rpm, it can be expected that the particles will start to struggle sliding over each other. This is due to the surface properties of the particles, in particular the surface area. In other words, viscosity is anticipated to change as the rate of agitation is varied. A similar situation applies regarding the effect of particle size on viscosity. At the same solids content, smaller particles can provide a larger surface area, and hence their effect on suspension viscosity will be more noticeable. In the manufacturing of pharmaceutical products, it should be kept in mind that properties such as particle size, shape, and size distribution can vary depending on the source of the material. For oral dosage forms that require a wet massing step, these particle changes can have significant effects and even change the rheological properties of the wet mass to where it becomes infeasible to process [1].
The physical stability of most formulated pharmaceutical suspensions is enriched by the addition of hydrocolloids or synthetic polymers that modify the vehicle viscosity. These materials are generally long molecular weight polymers such as guar gum, methyl cellulose, hydroxypropyl methylcellulose, and polyacrylics; often referred to as rheology modifiers or viscosity enhancers. Some of these materials such as methylcellulose help stabilize the suspension by increasing viscosity and by decreasing interparticle attraction which helps to prevent aggregation and caking over time [2]. Additionally, a combination of two or more of these ingredients in a dosage form is often seen to help enhance their individual effects [3]. Inorganic substances such as clays are also commonly employed as gelling agents to alter the viscosity of a formulation. One example is the use of magnesium aluminum silicate, an anionic clay, which can interact with polymers such as sodium alginate and chitosan to change their aqueous flow characteristics [4,5]. Similar to how solid particles are characterized, polymers are described by certain parameters, most notably their molecular weight and morphology. One may again expect changes in viscosity when these factors are varied.
Going back to water as our standard suspension vehicle, we can make water change its viscosity by adding more content into it such as the addition of solid particles to make a suspension and polymers to stabilize the suspension. When water contains variable amounts of suspended solids and polymers it will then lose its ability to maintain a constant viscosity over a wide range of shear rates. As such, water behavior becomes unpredictable and it is therefore necessary to perform rheological measurements to better understand the behavior of the suspension being formulated.
In manufacturing, having a complete rheological understanding of the material being processed is important to verify the equipment can effortlessly handle the job and perform it in an accurate and reproducible manner. In the liquid-filling of hard gelatin capsules, having an optimized solution viscosity is imperative to proper performance and can be manipulated by changing the concentration of the dispersed phase [6]. Rheology is also very important in the manufacturing of topical products. For example, imagine that a pump is being used to fill empty tubes with a semi-solid material such as a toothpaste or ointment. It will be necessary for fast production to have a predictable and smooth filling process and therefore a material that cooperates effectively when subjected to the high speed of pumping. If you know beforehand the behavior of the semi-solid material at varying pump speeds this process will occur with little complication. In this application, the product should desirably have a low viscosity at high pump speeds (shear rates) but be able to quickly recover and return to a higher viscosity upon standing. This is because a topical product should easily flow from the tube when a patient applies force and spreads on the medication, yet it should return to a sufficient viscosity as to remain on the skin and not flow off after application. Patient acceptability and therapeutic effectiveness of the semisolid product will vary according to how the flow property (spreadability) of the product changes with how fast or how slow the patient spreads it over her/his skin. Rheology is not only important to topical semi-solid products but to other innovative applications including parenteral therapies. For example, colloidal gels loaded with drugs have been studied as an injectable delivery system for use as an alternative to invasive surgery. Such gels are intended to accelerate the healing process and can be used as bone filler with shear thinning properties [7].
There is one additional factor that must not be overlooked in all the previous examples, the element of time. We all may have experienced how certain finished products display different behavior over time. So why do these changes occur? This happens because the rheological behavior of certain materials is time-dependent. In other words, they need time to adapt themselves to a new stress-induced flow condition. Time can also change the flow behavior of a product due to other factors such as excipient interactions, crosslinking, and polymer thermodynamic mobility. Time becomes a crucial factor most often when dealing with more complicated high-solid or high-polymer content suspension systems.
The viscosity of water doesn’t change with shear rate, however it changes depending on the contents it holds. This is similar to how blood serum does not change its viscosity significantly until the contents of red and white blood cells, proteins and other dissolved materials are added forming whole blood. Changing from a single to a multiphase system is what causes a change from a Newtonian to a non- Newtonian flow behavior. Factors that could cause such a transition are summarized in Figure 2. These factors include properties related to the solid particles in the dispersed phase (e.g. size, shape, aspect ratio, porosity, aggregation), the properties of rheology modifiers (e.g. one or multipolymer system, short or long chain polymer, crosslinked chains), and the nature of the suspension system itself (i.e. whether it’s diluted or concentrated). Any of these factors can to a lesser or greater extents force a Newtonian fluid to behave non-Newtonian. Without any of these, the viscosity of blood serum or water in effect would not change, except under the condition of changes in temperature which can easily be controlled or predicted (Figure 2).
Figure 2: Factors Affecting a Change from Newtonian to non-Newtonian Flow: large spherical particle (1), high aspect ratio particle (2), porous particle (3), small particle (4), high surface area particle (5), aggregated particles (6), plate-like particles (7), low solid suspension (8), high solid suspension (9), one polymer system (10), multipolymer system (11).
For instance with low solid content suspensions, changes in shear rate will not change the flow behavior and hence viscosity of the suspension system; however at higher solid content, particles will tend to see each other more frequently and have a better chance at interacting which can result in particle aggregation. The effect of increased interaction and aggregation causes the viscosity of the suspension to rise. When under no or low shear conditions, the intermolecular forces holding particles of an aggregate together are strong enough not to be overcome by the weak shear forces. However, as shear rates increase the individual particles of the aggregate will give up their intermolecular forces and start to break apart and align in the direction of increased shear. This loss or decline in resistance to flow results in a decreased viscosity of the fluid. This shear-thinning behavior of a fluid is referred to as pseudoplasticity. In a concentrated dispersion system, aggregates will tend to form or disappear when under low and high shear conditions respectively. This behavior is shown visually in Figure 3. Pseudoplasticity or shear thinning is the most common rheological behavior of the multiphase (heterogeneous) pharmaceutical dosage forms as can be seen in high solid creams and ointments. Time is again another factor that must be taken into consideration with regards to changes in the structure of the suspended particles. This is because certain systems exposed to a fixed shear rate over time will gradually break down when the thermodynamic status of the aggregated system is not kinetically stable and all that is needed for the system to get into a stable form is time (thixotropy). It has been shown that a mixture of polymers each individually exhibiting pseudoplastic behavior can be used together to create thixotropic behavior that is otherwise absent with the individual components [8] (Figure 3).
As the concentration of solid particles in a suspension increases, the system becomes progressively chaotic as more aggregates form as the result of reduced stability of the system and increased particle flocculation. There are now aggregated particles and not isolated particles which have to react to the changes in external forces. The fluid behavior associated with this is called dilatancy, and generally occurs in high solid content pharmaceutical suspensions (over 40% solid content), clay slurries, wet cement, candy compounds, corn starch in water, and sand/water mixtures (Figure 4). With all these, as shear rate increases, viscosity of the suspension increases as well. In other words, a dilatant pharmaceutical composition will remain stable at high shear condition. This same effect can occur with time, which is called rheopexy. If a pharmaceutical composition contains reactive excipients, reaction between these excipients will develop and proceed with time, causing an increase in viscosity. Dilatancy and rheopexy behavior are rare in pharmaceutical formulations.
If aggregation is responsible for the change in flow from a Newtonian to a Pseudoplastic and Dilatant flow behavior (Figure 5), factors affecting aggregation should also be accounted for. For instance, a particle with high aspect ratio (tubular or cylindrical) can potentially provide the same non-Newtonian behavior at lower concentrations that a particle having a low aspect ratio can at higher concentrations.
Polymers, particularly those of high molecular weight, can be deliberately added to pharmaceutical suspensions to enhance stability. A product’s rheological properties can be a good indicator of a products stability and shelf life. For example, creams that show more elastic rheology will often have longer stability and resist separation [9]. There are many examples of polymers found in pharmaceutical products that help control the viscosity, rheology, and hence stability of the final drug products as shown in Table 1. In oral tablets and transdermal patches, the role of the polymer is instead to provide appropriate release profile by changing the rheology and viscosity of the drug product either within the dosage form (patches) or in vivo (oral tablets) (Table 1).
| Dosage Form | Drug Product and Polymer Used |
|---|---|
| Gels | Retin-A Micro® (carbomer 974P (0.04%), carbomer 934P (0.1%) Benzamycin® Pak (hydroxypropyl cellulose, carbomer 934) Differin® (carbomer 940, poloxamer 124) |
| Creams | Tazorac® (carbomer 934P, carbomer 1342) Estrace® (hypromellose 2208 (4000 cps)) Bactroban (cetomacrogol 1000, mineral oil, xanthan gum) |
| Lotions | Elocon® (hydroxypropylcellulose) Cutivate® (propylene glycol, cetomacrogol 1000) |
| Pastes | Aphthasol® (gelatin, pectin, sodium carboxymethylcellulose) Kenalog® in Orabase /Oralone® (gelatin, pectin, and sodiumcarboxymethylcellulose) |
| Nasal Sprays | Flonase® (microcrystalline cellulose and carboxymethylcellulose sodium) Astepro® (hypromellose) Lazanda® (pectin) |
| Suspensions | Omnicef® (xanthan gum, guar gum) Augmentin® (xanthan gum, hypromellose) Carafate® (methylcellulose , microcrystalline cellulose) Indocin® (tragacanth) Ilevro™ (propylene glycol, carbomer 974P, guar gum, sodium carboxymethylcellulose) |
| Oral Tablets | Opana® ER (original formulation) (TIMERx®-N (xanthan gum and locust bean gum) Aldoril® (cellulose, ethylcellulose, guar gum, hydroxypropyl methylcellulose, magnesium stearate, propylene glycol) Arimidex (hydroxypropylmethylcellulose, polyethylene glycol, povidone, sodium starch glycolate) |
| Transdermal Patches | Lidoderm® (polyacrylic acid, polyvinyl alcohol, sodium carboxymethylcellulose, sodium polyacrylate) |
Table 1: Example of hydrophilic rheology modifying polymers used in drug products [10].
The key to enhanced stability is the extent of interaction between the polymeric stabilizer and the aqueous vehicle. The greater this interaction, the more stabilized the suspension will be. Polymers of high molecular weight and composed of water-loving functional groups can greatly help to achieve this goal. However, the interaction of these polymers with an aqueous medium will crucially depend on the nature of the aqueous medium. Factors such as pH, ionic strength, temperature, and addition of other organic solvents in the medium can alter the polymer-water interactions. To better help describe the effect of these factors, we offer the following case studies where a formulation scientist decides to use different excipients as a rheology modifier to enhance the stability of a drug suspension [10,11].
Polyacrylic acid as the rheology modifier for a suspension dosage form at a pH of 2
Polyacrylic acid is an anionic synthetic polymer, and similar to its hydrocolloid counterparts such as alginic acid and others that contain carboxyl groups, it enhances aqueous viscosity at higher pHs as shown in Figure 6; the cationic polymers do just the opposite. Therefore, given the fact that the pKa of polyacrylic acid is greater than 4, polyacrylic acid will be in a non-ionized form at suspension pH of 2. In the fully non-ionized form, weak acidic polymers such as polyacrylic acid will have limited solubility in an aqueous medium and therefore not significantly enhance viscosity (Figure 6).
Methyl cellulose as the rheology modifier for a suspension experiencing heat shock during its lifetime
Methyl derivatives of cellulose such as methyl cellulose and hydroxypropyl methylcellulose have different levels of methyl substitution and make aqueous solutions containing them sensitive to temperature. This is due to the fact that aqueous solutions of these polymers can undergo a sol-gel transition at certain temperatures that causes a drastic change in polymer solubility and hence viscosity [12]. For methyl cellulose solutions, this transition occurs at temperatures near 50°C. Therefore, if a suspension containing methyl cellulose is expected to undergo and withstand a thermal shock during any stage of its life time, the suspension stability will be compromised. Substitution of methyl with hydroxypropyl groups, such as that found on Hydroxypropyl Methyl Cellulose (HPMC), inhibits the gelation process at lower temperatures and the sol-gel transition is forced to occur at higher temperatures [13]. The inclusion of drug and other excipients can also alter the gelation temperature of HPMC solutions, an example being when the addition of nicotinamide increases both the viscosity and thermogelation temperature of HPMC aqueous solutions [14]. Chitin solutions have also been shown to be thermoresponsive, they are in solution form at low temperature, but become gel at the physiological temperature. More interestingly, the gelation temperature is concentration dependent, and it occurs at lower temperature when chitin concentration is higher [15].
Since viscosity of aqueous solutions is much greater when beyond the sol-gel transition temperature, care must be taken to avoid reaching temperatures near such transitions during manufacturing and storage. Temperature sensitive polymers can impart to a suspension an inverse thermoresponsive property where higher temperatures cause gel formation and higher aqueous viscosity. On the other hand, materials such as agar and gelatin can provide greater viscosity in water at lower temperatures compared to higher temperatures where they become soluble. These polymers provide the suspensions containing them a direct thermoresponsive property, where the suspension becomes less viscous at higher temperatures (Figure 7).
Polyacrylic acid as the rheology modifier for a cationic drug suspension
Since polyacrylic acid is an anionic polymer, one can expect strong intermolecular interactions between the negatively charged carboxyl groups of the polymer and the cationic drug. As such, the suspension dosage form will most likely lose its stability due to polymer-drug binding. Of course, this interaction is pH sensitive and can occur to a lesser or greater extent at different pHs.
Combination of alginic acid and chitosan as rheology modifiers for a suspension dosage form
The two polymers chosen are noticeably different in terms of charge; alginic acid being anionic and chitosan cationic. Although each can provide great viscosity in an aqueous medium, they will lose their viscosity modifying potential due to inter-polymer interactions if combined. Apparently, the ratio of the two polymers and the pH will critically affect this interaction. On the other hand, interaction of anionic and cationic polymers to form gels can be used in controlled drug delivery. For instance, peptides can be released in a controlled manner from PLGA nanoparticles coated with negatively charged alginate and positively charged chitosan [16].
Carboxymethyl cellulose as a rheology modifier in the presence of the traces of calcium, aluminum or iron
The carboxyl groups of carboxyl-substituted methyl cellulose are sensitive to cations including calcium, aluminum, and iron that can be found in other excipients used in the dosage form. This is true for other polymers containing carboxyl groups in the formulation as well. In the presence of cationic trace ions, a complexation between the ions and the carboxylate moieties is likely to occur. This results in a reduced hydration of the carboxylate groups and hence reduced viscosity of the suspension. Over sufficient time and at low polymer concentration, the polymer will fully precipitate in the suspension with no effect on viscosity.
Sodium carboxymethyl cellulose as a rheology modifier in a saline suspension vehicle
Osmotic forces are a big contributor in promoting interaction of polymers with water. Polymers such as sodium carboxymethyl cellulose when in water can provide osmotic pressure, and attract water until they become stable. If the polymer is used in an aqueous vehicle based suspension containing sodium, the osmotic forces would become suppressed, causing reduced viscosity. This is similar to how cells interact in water with different tonicity. More importantly, the osmotic pressure will be more suppressed if the suspension contains higher valence ions such as calcium or iron. Ions (salts), ions in the presence of a complexable polymer, and use of oppositely-charged polymer systems can cause instability, reduced polymer solubility, and polymer precipitation which results in reduced viscosity as shown in (Figure 8).
Combination of locust bean gum and xanthan gum as rheology modifiers
Xanthan gum forms a gel once it is mixed with locust bean gum; this combination has been used as an innovative TIMERx delivery technology [17]. The combination of locust bean gum and xanthan may not necessarily be good for other purposes such as enhancing viscosity. Although both can build great viscosity in water if used separately, the combined form fails to do so for the same reasons they are advantageous in controlled delivery. This is due to synergistic intermolecular polysaccharide chain aggregation that occurs causing the two polymers to form a gel instead of maintaining their solubility in water as individual entities.
High swelling crosslinked polymer as a rheology modifier along with a weak buffer
Pharmaceutical dosage forms may contain crosslinked hydrophilic excipients with the ability to swell in an aqueous medium causing enhanced viscosity. While non-ionic excipients may swell independent of pH, ionic excipients change their size (swell or shrink) when the pH of the aqueous medium changes as shown in Figure 9. For example, the anionic structure of crosslinked acrylic acid polymers in aqueous solutions will expand and gel when neutralized at higher pH values causing increased viscosity, whereas in an acidic environment viscosity is decreased [18]. A topical azelaic acid product (Finacea®) for the treatment of rosacea is formulated using carbomers (crosslinked polyacrylic acid) that are neutralized using sodium hydroxide in the final step to produce a clear and stable gel [19]. A change in pH can also be caused by loss of buffer, or inadequate buffer capacity, which put the polymer chains in a harsh environment causing them to fail (Figure 9).
Addition of non-solvent to a suspension having a rheology modifier
It was decided that a small amount of ethanol be added into the suspension dosage form to help enhance the formulation. However, this little alcohol may be sufficient in concentration to halt solubility, swellability and hence the viscosity enhancing ability of the rheology modifier. For instance, a polyacrylic acid system can stop building up viscosity at much lower alcohol concentration than a polymer such as methyl cellulose. The more hydrophilic (having very high HLB or hydrophilic lipophilic balance) the polymer is, the more sensitive it will become to the presence of alcohol in the suspension system.
Flow behavior of a suspension system is not only dependent on the dispersed phase (particle size, shape, morphology, concentration), but also crucially dependent on the rheology modifiers which are currently used in pharmaceutical suspensions to enhance their stability. Particle factors along with polymer factors can act synergistically to drastically change the behavior of a Newtonian solution or suspension to an extremely non-Newtonian suspension with unpredictable flow and stability property. This requires great care in selecting excipients and great knowledge and use of analytical equipment to evaluate the flow and stability of suspension systems.