Current Synthetic and Systems Biology
Open Access
ISSN: 2332-0737
ISSN: 2332-0737
Review Article - (2022)Volume 10, Issue 6
Many studies have been concentrated on the degradation of toxic organic compounds in waste water via photo catalysis of various semiconductors. It has attracted great attention in modern science because of its potential in solving many current environmental problems such as air and water pollution. The common photo catalysts are primarily nano composite metal oxides, are known to be good photo catalysts for the degradation of several environmental contaminants due to their high photosensitivity, stability and non-toxic nature. There are different approaches for the synthesis of nanomaterials: Top down and bottom up approaches. Top-down approach is best approach and refers to successive cutting of a bulk material to get nano sized particles. These applications have their interest in controlling particle size, particle shape, size distribution, particle composition and degree of particle agglomeration. Some nanoparticles have large band-gap which leads to high recombination rate of photo generated electron-hole pairs limits their utilization for photo catalytic applications. More recently, significant efforts have also been made to develop new or modified semiconductor photo catalysts that are capable of using visible-light (λ=400 nm-700 nm) including semiconductor coupling, metal ion doping, nonmetallic element doping, and sensitization with organic dyes. Coupling of two semiconductor nanoparticles with different band gap widths has been demonstrated in many studies as one of the most effective ways to reduce the recombination of electron-hole pairs and consequently, achieving a higher photo catalytic activity. Moreover, the ternary nano composites have high visible light photo catalytic activity and organic dyes can be decomposed efficiently, implying the higher photo catalytic activity of the ternary nano composites.
Coupling; Doping; Nanocomposite; Photocatalyst; Pollutant
Dye effluents from textile industries create severe environmental pollution problems by releasing toxic and potential carcinogenic substances into the aqua sphere. Human life is fully depending on water for agricultural and industrial activities. Wastewater treatment is a hot topic in water technology as well as in environmental chemistry. Therefore, it is more essential to develop financial and effective methods for dye wastewater treatment. The conventional technologies currently used to degrade the color of the dye contaminated water includes primary (adsorption, flocculation), secondary (biological methods), and chemical processes (chlorination, ionization) [1]. However, these techniques are non-destructive, since they only transfer the non-biodegradable matter into sludge, giving rise to new type of pollution, which needs further treatment [2].
The Active Oxidation Process (AOP) technique has drawn considerable attention from various quarters of scientific community as it is easy to handle and produces significantly less residuals as compared to the classical approaches. Amongst the many techniques employed in the AOP approach are the UV photolytic technique photo-fenton process, zonation process, sonolysis, photo catalytic approach; and the radiation induced degradation of dyes. Recently, lots of studies have been concentrated on the degradation of toxic organic compounds in waste water via photo catalysis of various semiconductors [3-9].
Nanoparticles have emerged as sustainable alternatives to conventional bulk materials, as robust, high surface area heterogeneous photo-catalysts and catalyst supports [10]. The nano-sized particles have high surface to volume ratio which increase the exposed surface area of the active component of the catalyst, enhances the contact between reactants and catalyst [11]. Therefore, surface area plays an important role in the photo catalytic activity, due to that focus had been shifted towards the semiconducting nanomaterial because of their high surface to volume ratio [12]. It is required for a contaminant molecule to be adsorbed on to the surface of photo catalyst for the redox reactions (oxidation and reduction) to occur for the complete degradation/mineralization of the contaminant [13]. Nanoparticles that have a large band-gap semiconductor with unique structure and properties have been applied in photo catalysis, but the high recombination rate of photo generated electron–hole pairs limits their utilization for photo catalytic applications [14]. Therefore, efforts have been paid to improving the photo catalytic performance of nanoparticles via different strategies, such as synthesis of nano rod arrays, one-dimensional nano rods, combination with other components, modification of metal oxide nanoparticle by non-metal doping, addition of transition metals, use of binary and ternary semiconductors [15-20].
The coupled semiconductor materials have two different energy level systems which play an important role in achieving charge separation. Coupling of two semiconductor nanoparticles with different band gap widths has been demonstrated in many studies as one of the most effective ways to reduce the recombination of electron-hole pairs and consequently, achieving a higher photocatalytic activity. These systems also exhibit higher degradation of organic pollutants. Several narrow band gap metal oxides providing new opportunities to harvest photons in visible region have been coupled to fabricate visiblelight photo catalysts. Thus far a number of coupled systems such as the Cu2O-ZnO and have been reported to exhibit visible-light photo catalytic activity to a certain extent. For example, in coupled system of Cu2O-ZnO, the Conduction Band (CB) level of Cu2O relatively higher than that of ZnO. Hence the photo generated electrons are transferred to the CB of ZnO, and these energetic electrons can initiate many reduction reactions. We classified this system as "Type-A hetero junction”, which will be eligible for the reduction reaction or partial decomposition of organic pollutants. However, CO2 evolution resulting from complete oxidation of organic pollutants will be slow, due to the unavailability of the OH radicals on the ZnO surface.
Contrarily, if the Valence Band (VB) level of the sensitizer is located lower than that of nanoparticle, the visible light sensitization can induce the hole-transfer from the sensitizer to nanoparticle. As a result, holes can be generated in the VB of metal oxides nanoparticle, initiating in turn various oxidation reactions. Considering the powerful oxidative ability of the holes in the VB of ZnO, efficient and complete oxidation of organic compounds is expected to this system (denoted as Type-B heterojunction) under visible-light for example Ag3PO4/ZnO heterojunction.
Recently, semiconductor Ag3PO4 has attracted considerable attention as a potential visible light photo catalyst with a band gap of 2.45 eV. It’s Conduction Band (CB) and Valence Band (VB) edge potentials are 0.45 eV and 2.9 eV, respectively, and its VB potential is lower than that of ZnO with 2.6 eV. Therefore Ag3PO4 is considered to be an appropriate sensitizer to improve photo catalytic activity in the Ag3PO4/ZnO system, in which ZnO works as a substrate, while the role of Ag3PO4 is a sensitizer absorbing visible light.
The ternary nano composites have high visible light photo catalytic activity and organic dyes can be decomposed efficiently, implying the higher photo catalytic activity of the ternary nano composites for example, ZnO/ZnS/CuS ternary nano photo catalyst under visible light compared to the ZnO nanoparticles and ZnO/ZnS binary Nano composite. This is due to the reduce the recombination of photo generated electrons and holes, because the interface between the phases can act as separation site for the photo generated electron and holes due to the difference in the energy levels of their conduction band and valance band, thus the visible light photo catalytic activity of ZnO/ZnS/CuS can be enhanced.
Synthesis method of nano composites
There are two general approaches for the synthesis of nanomaterials. Top down and bottom up approaches. Top-down approach refers to successive cutting of a bulk material to get nano sized particles. Bottom up approach refers to the buildup of a material from atoms or molecules. Both the top-down and bottom-up approaches may be carried out in gas, liquid or solid states, with a variety of different applications. These applications have their interest in controlling particle size, particle shape, size distribution, particle composition and degree of particle agglomeration. Before discussing the synthesis, processes adopted in the present investigations, a brief review of some of the well-known top-down and bottom-up methods of synthesis are discussed in the following sections.
Top-down method
Top-down approach involves the breaking down of the bulk material into nano sized structures or particles. Top-down synthesis techniques are extension of those that have been used for producing micron sized particles. These approaches are inherently simpler and depend either on removal or division of bulk material or on miniaturization of bulk fabrication processes to produce the desired structure with appropriate properties. The biggest problem with the top-down approach is that it introduces internal stress, surface defects and contaminations. For example, nanowires made by lithography are not smooth and may contain a lot of impurities and structural defects on its surface. Examples of such techniques are high-energy wet ball milling, electron beam lithography, atomic force manipulation, gas-phase condensation, aerosol spray, etc.
Gas phase condensation: The simplest fashion to produce nanoparticles is by heating the desired material in a heat resistant crucible containing the desired material. This method is appropriate only for materials that have a high vapor pressure at the heated temperatures that can be as high as 2000℃. Energy is normally introduced into the precursor by arc heating, electron beam heating or Joule heating. The atoms are evaporated into an atmosphere, which is either inert (e.g. He) or reactive (so as to form a compound). To carry out reactive synthesis, materials with very low vapor pressure have to be fed into the furnace in the form of a suitable precursor such as organometallics, which decompose in the furnace to produce a condensable material. The hot atoms of the evaporated matter lose energy by collision with the atoms of the cold gas and undergo condensation into small clusters via homogeneous nucleation. In case a compound is being synthesized, these precursors react in the gas phase and form a compound with the material that is separately injected in the reaction chamber. The clusters would continue to grow if they remain in the supersaturated region. To control their size, they need to be rapidly removed from the supersaturated environment by a carrier gas. The cluster size and its distribution are controlled by only three parameters: (1) The rate of evaporation (energy input), (2) The rate of condensation (energy removal), and (3) The rate of gas flow (cluster removal) (Figure 1).
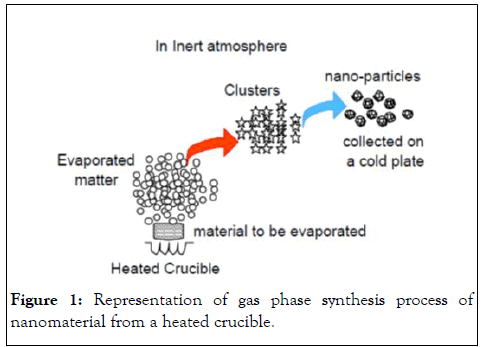
Figure 1: Representation of gas phase synthesis process of nanomaterial from a heated crucible.
Because of its inherent simplicity, it is possible to scale up this process from laboratory (mg/day) to industrial scales (tons/day).
Bottom-up method The alternative approach, which has the potential of creating less waste and hence the more economical, is the ‘bottom-up’. Bottom-up approach refers to the buildup of a material from the bottom: Atom by atom, molecule by molecule, or cluster by cluster. Many of these techniques are still under development or just beginning to be used for commercial production of nano powders. Organometallic chemical route, revere-micelle route, sol-gel synthesis, colloidal precipitation, hydrothermal synthesis, template assisted sol-gel, electrode position etc, are some of the well-known bottom up techniques reported for the preparation of luminescent nanoparticles.
Colloidal precipitation: The main advantage of this method is the use of non-toxic precursors and water are used as solvent. The synthesis of ZnS nanoparticles was carried out by aqueous chemical method using zinc chloride (ZnCl2) and sodium sulfide (Na2S) as source materials. The entire process was carried out in distilled water for its inherent advantages of being simple and environment friendly. All steps of the synthesis were performed at low temperature and ambient conditions. In a typical preparation, solution of 1 M zinc chloride was prepared in 100 mL of deionized water and then the solution of 1 M sodium sulfide was added drop wise to the solution which was kept on stirring using a magnetic stirrer at 70°C, which resulted in formation of ZnS nanocolloid. The nanoparticles were collected by centrifugation at 2000 rpm for 15 minutes and further purification was made by ultrasonic bath. The resultant product was finally dried and used for photocatalytic degradation of dye.
Sol-gel synthesis: Sol-gel technology is a low-temperature method of preparing inorganic materials by chemical routes. Many researchers had paid attention in synthesizing new photocatalysts. Sol-gel techniques have been used for many years to synthesize nanoparticles. In a typical technique, a sol (nanoparticles dispersed in a solvent by Brownian motion) is prepared using a metal precursor (generally alkoxides, acetates or nitrates) in an acidic or basic medium. The three main steps in this process are hydrolysis, condensation (sol formation) and growth (gel formation). In brief, the metal precursor hydrolyzes in the medium and condenses to form a sol, followed by polymerization to form a network (gel) (Figure 2).
Figure 2:TiO2 nanoparticle mediated mesoporous film preparation by sol-gel processing.
This method has been used to synthesize II-VI and IV-VI nanoparticles, such as CdS, ZnS, and PbS. As an example, ZnS nanoparticles have been prepared by mixing solutions of Znacetate in alcohol and sodium sulfide, followed by control aging in air. In addition, TiO2/ZrO2/SiO2 ternary composite was synthesized using sol-gel method from (Ti (OC3H7)4, Zr (OC3H7)4, tetraethoxysilane Si (OC2H5)4, in the presence of Acetylacetone (AcAc,) as a complex form for the determination of its optical conductivity. The ternary SnO2-ZnO-ZnWO4 nanocomposite was similarly prepared by a sol-gel route from Zn(AcO)2, SnCl4, and Phosphotungstic Acid hydrate (PTA) (H3PW12O40 × H2O) solutions for the evaluation of photocatalytic activity of the sample on the decomposition of 4-nitrophenol and partial oxidation of 4-methoxybenzyl alcohol to p-anisaldehyde.
Among the various methods, sol-gel method has attracted more attention because of low process cost, easy control of composition, suitable for scale-up and relatively low calcinations temperatures. The main disadvantages of the sol-gel process include a broad size distribution and a high concentration of defects. Therefore, this synthesis technique is used sparingly.
Coprecipitation: Coprecipitation reactions involve the simultaneous occurrence of nucleation, growth, coarsening, and/or agglomeration processes. Precipitation reactions exhibit the following characteristics: The products are generally insoluble species formed under conditions of high super saturation. Nucleation is a key step, and a large number of small particles will be formed. Secondary processes, such as Ostwald ripening and aggregation, dramatically affect the size, morphology, and properties of the products. The super saturation conditions necessary to induce precipitation are usually the result of a chemical reaction. For example the CeO2/CuO/ZnO metal ternary composite was prepared by co-precipitation of their carbonates from the aqueous solutions of metal salts such as, Zn (CH3COOH)2.2H2O, Cu (NO3)3.6H2O, Ce2 (SO4)3.4H2O and a solution of Na2CO3 used for the degradation of methyl violet dye and anti-bacterial activity. Similarly, ternary composite oxide catalysts of CuO/Co3O4-CeO2 with wide temperature-window was synthesized from NaOH, Co (NO3)2.6H2O and Ce (NO3)3.6H2O solutions for the preferential oxidation of CO in H2 rich stream.
Hydrothermal synthesis: Hydrothermal synthesis is an attractive method due to its simplicity and productivity. In addition, under supercritical condition, it leads to the formation of nanoscale products, which cannot be obtained by classical routes. The hydrothermal method is a very effective method for the preparation of inorganic nanomaterials, such as oxides, sulfides, phosphates, zeolites and diamond. The particle sizes and their distributions, phase homogeneity, and morphology, can be well controlled in this method. In solvothermal/ hydrothermal process the decomposition of the precursors at a particular solvent depends on the temperature and pressure inside the reaction vessel.
For example, ZnO nanorods were prepared by a solvothermal method from AgNO3 and Na3PO4 solutions. In addition, ZnO/CuO/ZnAl2O4 ternary composite was prepared by a Polyethylene Glycol (PEG) assisted hydrothermal synthetic method using the glucose based carbonaceous materials as template and Cu(NO3)2·3H2O, Al(NO3)3·9H2O, Zn(NO3)2·6H2O and Polyethylene Glycol (PEG).
Chemical bath deposition method: The electron deposition method was carried out by a sequential chemical bath deposition method. For example, the TiO2/ZnO/CdS ternary hybrids were prepared by electron deposition method. In these method TiO2/ZnO binary nanocomposites was used as the starting material for the deposition of CdS thin films, the photocatalytic activities of TiO2/ZnO/CdS semiconductor composite was evaluated by using the photo degradation of Alizarin Red S (ARS) solution under UV photoirradiation.
Microemulsion method: The microemulsion is represented as a set of droplets randomly located on a three dimensional lattice, which can move and collide with each other. Each simulation begins with a random distribution of the two or three sets of microemulsion droplets, depending on the kind of nanoparticle: If the simulated nanoparticle is a simple one, the one-pot method is simulated by mixing equal volumes of two microemulsions, one containing the reactant A and other containing the reactant B, with the reaction A+B→P.
To simulate the synthesis of a bimetallic nanoparticle, three microemulsions are mixed, one containing the metal salt A, the second containing the metal salt B, and the third containing the reducing agent R. Zinc oxide nanostructures with, various morphologies were prepared by microemulsion method.
Incipient wetness impregnation method: Incipient wetness impregnation method is commonly used method for synthesis of heterogeneous catalysts. Typically, the active metal precursors are dissolved in aqueous or organic solution. Then the metal containing solution is added to a catalyst support the same pore volume as the volume of solution that added. The catalyst can be dried and calcined to drive off the volatile components within the solution, depositing the metal on the catalyst surface.
In-situ polymerization method: In-situ polymerization synthesis method is always used to synthesize the inorganic nanoparticle in polymer matrix and it happens between the inorganic colloid and the polymer monomer with the presence of the strong oxidant. The presence of the strong oxidant important for polymer monomers is polymerized with inorganic nanoparticles. This preparation method does not need high temperature because the polymerization always starts up with the temperature which is lower than 10℃. It is a simple and effective route to prepare nanocomposites. This method allows one-step fabrication of nanocomposites with in situ generated nanoparticles from corresponding precursors. In this case, the nanoparticles can be grown inside the polymer matrix. The advantage of this route is that it prevents particle agglomeration while maintaining a good spatial distribution in the polymer matrix (Figure 3).
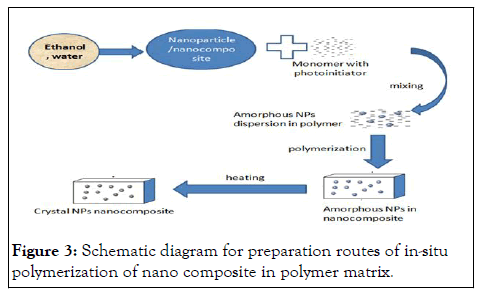
Figure 3: Schematic diagram for preparation routes of in-situ polymerization of nano composite in polymer matrix.
Approch to making semiconductors visible light active
Semiconductor photo catalysis has been intensively studied in recent decades for a wide variety of application such as hydrogen production from water splitting, water and air treatment. The majority of photo catalysts are, however, wide band-gap semiconductors which are active only under UV irradiation. In order to effectively utilize visible solar radiation, wide band-gap semiconductor photo catalyst must be modified.
A large number of metal oxides and sulfides have been examined as photo catalysts for hydrogen production and environmental application. The majority of the simple metal oxide photo catalysts, however, are primarily active under UV irradiation (λ<385 nm or Eg ≥ 3.0 eV), present in only a small portion of solar light. For example, ZnS and ZnO have wide band-gap energy of (3.72 eV) and (3.37 eV) respectively which prevents the utilization of visible light that accounts for most of solar energy. More recently, significant efforts have also been made to develop new or modified semiconductor photo catalysts that are capable of using visible-light (λ=400 nm-700 nm) including semiconductor coupling, metal ion doping, nonmetallic element doping, and sensitization with organic dyes.
Band gap modification by doping
Nitrogen doping: The substitution N doping generates a new band close to the VB of ZnO with which electrons from the valence band of ZnO makes a two-step transition to the conduction band using visible light. Density of states calculation made by conclude that substitution nitrogen species generate states just above the valence band maxima that can mix with O 2p valence states to narrow the band gap of semiconductors. Recent investigations indicate that the desired band gap narrowing of ZnO can be better achieved by employing nonmetal elements such as N, F, S, P and C. Such modified ZnO showed stronger absorption in the visible region owing to band gap narrowing and enhanced the degradation of organic pollutants under visible light irradiation, especially under solar light (Figure 4).
Figure 4: Visible light absorption by nitrogen doped ZnO.
Transition metal doping: ZnO has been doped with various transition metals. When these transition metal ions substitute for Zn2+ ions with tetrahedral O coordination in ZnO lattice, the band gap narrows by sp-d exchange interactions between conduction band electrons (CB made up of 4s 4p orbitals of Zn) and d electrons of these transition metals. High visible light activity has been observed with Co and Cu doped ZnO, with an increase in Co content in ZnO the absorption of visible light and content of surface oxygen vacancies increased. Co doped ZnO showed better visible light activity than Mn and Ni doped ZnO photocatalysts owing to comparatively better crystalline and narrower band gap.
Chromium doping: Chromium doped ZnS nanoparticles, with 0.05, 0.1, 0.2 and 0.3 mol% of Cr had been prepared using incipient wetness impregnation method. Kinetics of photo catalytic degradation of Methyl Orange (MO) dye catalyzed by synthesized nanoparticles was studied under UV and visible radiation. Effect of parameters such as dopant concentration, pH, and dye initial concentration on the photocatalytic degradation of MO dye were investigated. Photo catalytic degradation decreased with increasing dye initial concentration. Using 0.2 mol% Cr-ZnS as photocatalyst, the dye was degraded 74.28% and 65% under visible and UV radiation, respectively, at 5 hrs of the reaction. Higher photo catalytic degradation of dye under visible radiation than under UV radiation is attributed to red-shift of the absorption edge of the Cr-doped ZnS photo catalyst enabling it to harvest more photons in the visible light.
Surface modification via organic materials and semiconductor coupling
Dye sensitization: Sensitizing dye also used in the semiconductor with higher band-gap to change the electron transfer processes during photo catalytic reaction. The principle of photosensitization of a semiconductor is illustrated. The energy difference between the oxidation potential of the excited sensitizer and the conduction band of the semiconductor acts as a driving force for the charge injection process.
Dye sensitization has been demonstrated as a useful tool to induce visible light photo catalysis on the surface of wide band gap semiconductors like ZnS and ZnO which are otherwise inactive under visible light physical adsorption of dyes occurs through the weak Vander Waal’s interaction between the dye molecule and the surface of semiconductor. Dye sensitization facilitates electron transfer between the dye molecules and the host semiconductor.
Figure 5:Visible light activation of a wide band gap semiconductor by dye sensitization.
The dye sensitization process involves the excitation of dye molecules with visible light and the subsequent electron injection in to Conduction Band (CB) of a semiconductor (1) and (2), followed by a back reaction (3).

If the back reaction (3) is prevented, dye undergoes irreversible degradation (4) while photo injected electrons in conduction band form active oxygen radicals (5) yielding overall degradation of the sensitized dye.
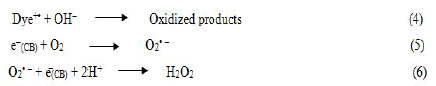
In sensitized degradation, catalytic activity depends on, the energy of the electron produced and the extent of its separation. A simple approach to suppress the back electron transfer is to produce a long distance charge separation. It had been clearly demonstrated that a wider separation of the electron and the oxidized dye enhances the catalytic efficiency by suppression of recombination.
The main requirements a dye molecule should fulfill in order to be considered as a good candidate for spectral sensitization of semiconductors can be summarized by the following points.
• Strong absorption across the entire visible spectrum (broad range of wavelength and high).
• Strong binding to the semiconductor surface (a group that can attach to the ZnS paste).
• Energy levels at the proper positions (LUMO high enough in energy for efficient charge injection and HOMO low enough for efficient regeneration).
• Rapid electron transfer to the ZnS in comparison to decay to the ground state of the dye.
• Stability over long period of exposure to sunlight.
• Low cost, simple and reproducible synthesis and purification.
• Small reorganization energy for excited and ground state electron transfer to minimize energy losses.
Semiconductor coupling: As reported by Rajesh, et al. ZnS-CdS photo catalyst was synthesized from zinc acetate, cadmium acetate and hydrogen sulfide as source materials. The semiconductor was prepared by taking known weight of salts of Zn and Cd and precipitating by passing H2S. The precipitate was allowed to settle down and further presence of precipitate was checked by passing more H2S in the supernatant solution. Then the precipitate was washed several times with distilled water and was allowed to dry at room temperature. The photo catalytic activity of the as synthesized photo catalyst was studied under visible light radiation for the photo degradation of crystal violet at different parametric effects. It was shown that optimum amount of ZnS-CdS catalyst was able to degrade crystal violet efficiently at optimum pH and initial dye concentration (Figure 6).
Figure 6:Visible light activation of ZnS by coupling with a narrow band gap semiconductor.
Recently, some studies also tried to improve the photo catalytic activity of wide band gap photo catalysts by coupling with different metal oxides systems. The Fe2O3-ZnO-MnO2 ternary composite coupled system has band gap energy of 2.53 eV. This band gap energy is narrow compared with band gap energy of ZnO that is 3.37 eV. This decreasing band gap energy is as the result of combining ZnO and Fe2O3 semiconductor nanoparticles with band gap widths. Semiconductor coupling is one of the most effective applications to reduce the electron-hole pair’s recombination. This because when the visible light hit the VB of combined semiconductor , CB electron can be ejected from the low band gap semiconductor (Fe2O3) to the band gap semiconductor (ZnO) and on the contrary, hole transfer can take place from the VB of high band gap semiconductor (ZnO) to the VB of low band gap semiconductor (Fe2O3). Additionally, the presence of electron accepter Mn (IV) could scavenge the excited electrons and altogether prevent the recombination of electron-hole pairs and the charge separation of the carriers. The ternary nano composites have high visible light photo catalytic activity and organic dyes can be decomposed efficiently, implying the higher photo catalytic activity of the ternary nano composites.
Heterogeneous photo catalysis has great potential for the mineralization of non-biodegradable pollutants in air and water. The majority of the simple metal oxide photo catalysts are primarily active under UV irradiation (λ<385 nm or Eg ≥ 3.0 eV), present in only a small portion of solar light. More recently, significant efforts have also been made to develop new or modified semiconductor photo catalysts that are capable of using visible-light (λ=400 nm-700 nm) including semiconductor coupling, metal ion doping, nonmetallic element doping, and sensitization with organic dyes.
Coupling of two semiconductor nanoparticles with different band gap widths has been demonstrated in many studies as one of the most effective ways to reduce the recombination of electron-hole pairs and consequently, achieving a higher photo catalytic activity. Several narrow band gap metal oxides providing new opportunities to harvest photons in visible region. Thus far a number of coupled system compounds showed significantly higher photo catalytic activity than pure nano composite, because the coupling of nano composite promoted the separation of electrons and holes. The ternary nano composites have high visible light photo catalytic activity and organic dyes can be decomposed efficiently, implying the higher photo catalytic activity of the ternary nano composites compared to the single nanoparticles and binary nano composite.
Citation: Ali AH (2022) Review on the Synthesis Method of Nano Composites and Approach to Making Semiconductors Visible Light Active. Curr Synthetic Sys Biol. 10:015.
Received: 01-Aug-2022, Manuscript No. CSSB-22-18624; Editor assigned: 03-Aug-2022, Pre QC No. CSSB-22-18624(PQ); Reviewed: 17-Aug-2022, QC No. CSSB-22-18624; Revised: 01-Nov-2022, Manuscript No. CSSB-22-18624(R); Published: 08-Nov-2022 , DOI: 10.35248/2332-0737.22.10.015
Copyright: © 2022 Ali AH. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.