
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Case Report - (2020)Volume 10, Issue 4
Objectives: To report atypical aspects of cardiovascular manifestations related to phostoxin poisoning and to propose
a therapeutic approach in the case of myocarditis induced by intoxication.
Patient and method: We report the medical observation of an atypical case of a patient admitted to the medical
intensive care unit then to cardiology department in university hospital Ibn Rochd of Casablanca for aluminum
phosphide poisoning.
Case report: A 30 -year-old man initially admitted to the emergency department resuscitation room and then to the
cardiology department, presenting with ventricular dysfunction at 30% ejection fraction following myocarditis due to
aluminum phosphide voluntary poisoning with left ventricular apical thrombus. Under treatment of heart failure,
associated with the administration of trimetazidine and anticoagulation, the patient recovered to 50% of LVEF after
fifteen days of treatment with thrombus dissolution. From a case reported in the literature on the impact of
trimetazidine in the evolution of myocarditis in phostoxin intoxication, we discussed the particular effect of
trimetazidine in the favorable evolution in our patient.
Conclusion: No specific drug has been established as an antidote for the phostoxin poisoning, Hence treatment
strategy is mainly supportive until the myocardial injury induced by the active metabolite (phosphine) subsides. Our
use of Trimetazidine for this patient with encouraging results needs further validation by others, as it may be a useful
adjunct to aggressive supportive care when needed alongside with other conventional treatments for heart failure.
Aluminium phosphide; Phostoxin; Myocarditis; Trimetazidine; Poisoning; Toxicology
Aluminium phosphide (ALP) is a fumigant pesticide widely used among farmers for stored cereal grains. Its use presents a danger both collectively and individually, particularly in developing countries where its use is not governed by strict regulations [1].
Let's not forget his voluntary use for a suicidal or criminal purpose.
Indeed, in Morocco, phostoxin® poisoning has been a major public health problem, as the product becomes a common cause of suicidal poisoning, due to his low cost and his availability [1,2].
Acute phosphide poisoning is a real emergency burden of heavy mortality requiring early and adequate care [1]. Lack of antidote, severe cardiac injury, cardiogenic shock following ingestion, all appears as responsible for high mortality rates [3].
Cardiac involvement is essentially a myocarditis which is in this case the consequence of a toxic reaction, and responsible for the various cardiovascular manifestations. It is often fatal and poses a problem of effective treatment [4,5]. The aim of our work was to report atypical aspects of cardiovascular events related to phostoxin poisoning and to propose a therapeutic approach.
We report the medical observation of an atypical case of a patient admitted to the medical intensive care unit of university hospital Ibn Rochd of Casablanca for aluminum phosphide poisoning.
A 30-year-old man was admitted to the emergency department – resuscitation room with confusion, profuse sweating, and vomiting.
Medical history reported by the case's relatives revealed intentional ingestion of 1 tablet of aluminium phosphide (Phostoxin*) three hours before admission.
Physical examination at the time of admission revealed, Glasgow coma Scale 13/15, cold extremities, blood pressure of 80/50 mmHg, pulse rate of 93 bpm, and oxygen saturation of 94% on room air (pulse oximetry).
Cardio pulmonary auscultation revealed clear lungs with no crackles and no additional cardiac murmur.
Chest X-ray was normal.
The electrocardiogram showed diffuse negative T waves (all leads except V1) (Figure 1).
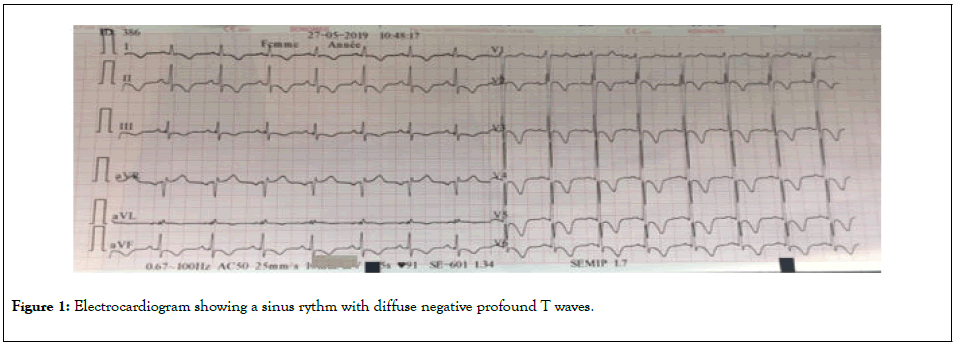
Figure 1: Electrocardiogram showing a sinus rythm with diffuse negative profound T waves.
Complete blood count showed: Hb: 15.3 g/dl, WBC: 4250/ mm3, PLT: 120000/mm3, Na+: 139 mmol/l, K+: 3.9 mmol/l, Urea/creat: 0.62 g/l /8.3 mg/dl; LDH: 433 U/l, CK: 206 U/l, CRP: 86.4 mg/l, Mg2+: 0.45 mmol/l, Troponins us at admission: 399 ng/l.
Intravenous inotropic agent was administrated, dobutamine 20 μg/kg/min, associated with high-concentration Oxygen mask ( 8l/min) with hemodynamic stabilization 6 hours later.
Initial hypo-magnesemia was treated intravenously by supplemental magnesium sulphate 2 mg.
On the next day, blood analysis showed troponin raised levels: 4099 vs. 399 ng/l initially; without electrical modifications.
Toxic screening confirmed aluminium phosphide intoxication.
Bedside echocardiogram showed: Global hypokinesia of the left ventricle with reduced ejection fraction of 30% and an non adherent thrombus in the apex of left ventricle measuring 24 × 13 mm (Figure 2).
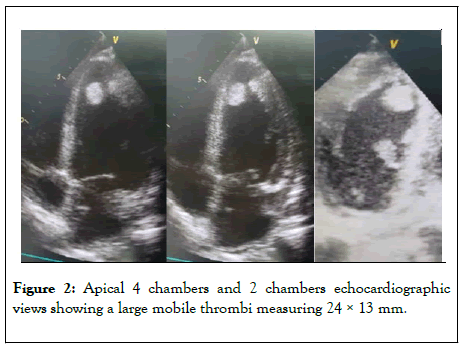
Figure 2: Apical 4 chambers and 2 chambers echocardiographic views showing a large mobile thrombi measuring 24 × 13 mm.
The right ventricle had a preserved systolic function with a TAPSE of 19 mm.
The patient was weaned from inotropes 48 h after admission and acute systolic heart failure treatment was initiated, using diuretics (furosemide 40 mg per day), conversion enzyme inhibitors (Ramipril 1.25 mg increased to 2.5 mg per day) betablockers (Bisoprolol 2.5 mg increased to 5 mg once daily), and spironolactone (25 mg a day) besides curative anticoagulation (Enoxaparin SC 0.6 ml twice daily).
Adjunction of Trimetazidine (35 mg twice daily) was started early on since the first day of admission.
Once transferred to Cardiology department, a coronary angiogram was performed showing normal epicardial coronary arteries and then left ventricle was evaluated by global longitudinal strain being at-6.2% (Figure 3).
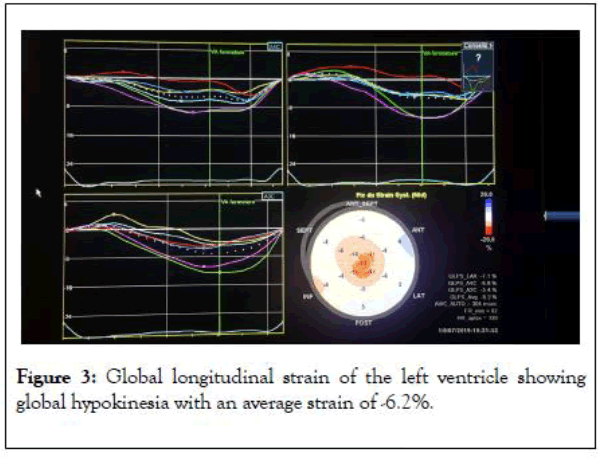
Figure 3: Global longitudinal strain of the left ventricle showing global hypokinesia with an average strain of -6.2%.
Patient’s follow-up showed improvement of the hemodynamic status with Blood pressure of 13/08 cmHg, biologic (troponin on the 15th day: 69 ng/l).
EKG controls showed persistence of T waves inversion even by the 15th day following admission.
Echocardiographic follow up parameters noticed that the left ventricle ejection fraction increased to 50% and the global longitudinal strain to –17.6% (Figure 4), after 15 days of ALP ingestion.
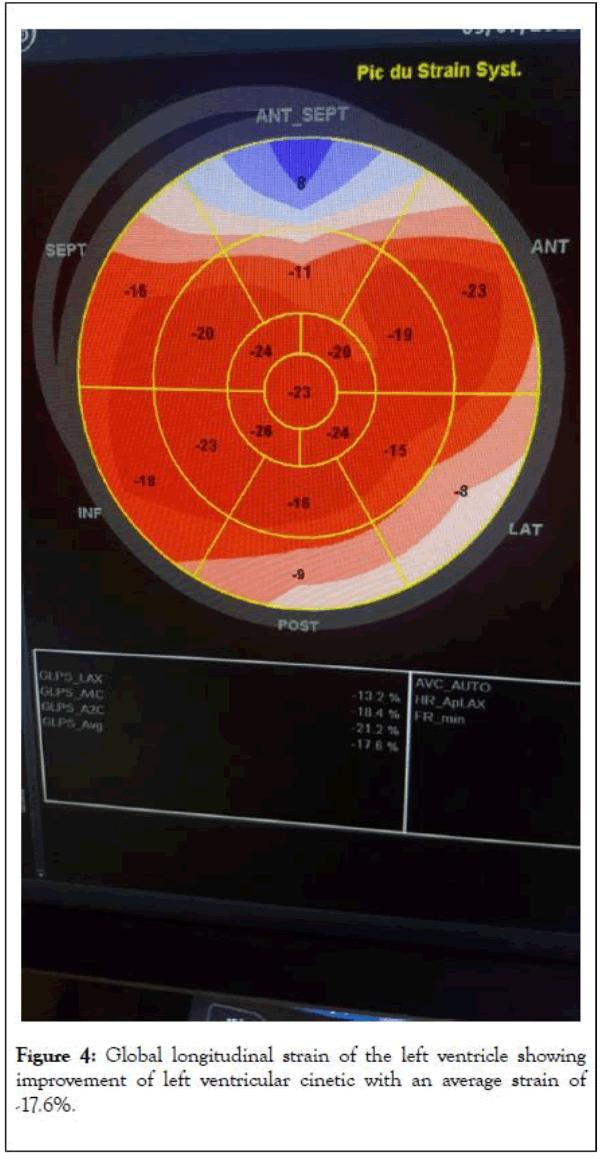
Figure 4: Global longitudinal strain of the left ventricle showing improvement of left ventricular cinetic with an average strain of -17.6%.
Regression of the intraventricular thrombus was noted starting from 7th day following ingestion with total disappearance on 15th day.
Aluminum phosphide poisoning is known worldwide, especially in developing countries, It is available on market and is purchased without any restrictions in some countries such as Morocco under trade names e.g. Phostoxin, or commonly known for “cereal tablets” in local shops.
It’s grayish tablet weighing about 3 g, containing 56% aluminium phosphide and 44% aluminium carbonate, capable of releasing 1 g of phosphine [6].
Lethal dose of ALP is 1-1.5 g. Deaths are reported even with a dose of 150-500 mg.
Following oral ingestion, AlP reacts with water and stomach acid to produce phosphine gas according to the following reaction:
AlP+3H2OAl(OH)3+HP3
Which account for the toxicity of this product.
Phosphine gas is rapidly absorbed by inhalation, or in the major part via the enteral route.
Phosphine leads to non-competitive inhibition of the cytochrome oxidase of mitochondria, blocking the electron transfer chain and oxidative phosphorylation, resulting in decreased ATP formation and reducing oxidative respiration by 70%, producing an energy crisis in the cells [7].
It further inhibits the antioxidant enzymes, catalase and peroxidase, decreasing the neutralization of reactive oxygen species products (ROS) leads to cellular hypoxia with likely multiorgan attack [8].
High level of cellular superoxide and peroxide radicals is related to a subsequent cellular damage by lipid peroxidation hence to mortality ; Decreasing the serum levels of these biochemical parameters to near normal level may be lead to survival as was the case for our patient.
Aluminium phosphate poisoning is often translated clinically by epigastric pain, nausea, vomiting and cardiogenic shock reflected as severe refractory hypotension [9].
The cardiovascular toxicity is well documented in literature as described in several case reports ; which explain the high mortality rates.
Cardiac manifestations of this poisoning are dominated by refractory hypotension, acute heart failure, myocarditis with electrocardiographic abnormalities (ECG), interesting ST segment and T wave and conduction defects [10].
Timed ECG changes was studied showing that sinus tachycardia is predominant during the first 3 to 6 hours following the ingestion, starting from the 6th hour ST-T changes occurs and conduction disturbances appear, while in the later period, represent the risk of fatal arrhythmias (ventricular tachycardia 40%, ventricular fibrillation 23.3% ) [11].
However few studies evaluated cardiac function by echocardiography with closed cardiac imaging follow-up, revealing dysfunction type of hypokinesia of the anterior wall of the left ventricle that was reversible over few days [12].
Our Patient experienced severe myocardial depression with a concordant ECG diffuse repolarization disorder (T wave inversion), with a high level of troponin, confirmed by echocardiogram showing an important reduction in the left ventricular systolic function with global severe hypokinesia.
Similar cases have been reported in literature with a marked decrease in the systolic ventricular function, with a pattern of global hypokinesia of the left ventricle walls in majority of cases and reversal of echocardiographic findings in aluminium phosphide poisoning survivors by the fifth day [13].
Concerning our case, the TTE control showed a gradual improvement in the left ventricular ejection fraction by the 15th day with an improvement from 30% LVEF to 50%.
To our knowledge, it’s rare the developing of left intraventricular thrombus in ALP poisoning with only one case reported [14]. Nevertheless, it is known that thrombi can develop as a result of myocarditis especially as the left ventricular ejection fraction is impaired. The particularity in our patient is the relatively rapid formation of the thrombus.
The danger of ALP poisoning lies in the absence of a specific antidote, Hence the management of ALP intoxication remains primarily supportive.
Different therapeutic modalities was reported with varied degrees of success, but with little evidence base, including magnesium sulphate, N-acetyl cysteine, intra-aortic balloon pump, and extracorporeal membrane oxygenation as an assist device.
However few are studies who were interested in the utility of Trimetadizine in alumimium phosphate poisoning with only one case report [15]. Trimetazidine is an anti-ischemic drug that is protective of the myocardium due to its preservation of oxidative metabolism, acting by decreasing the cellular accumulation of calcium and improving intracellular ATP levels.
These mechanisms may explain the favorable effect of trimetazidine in our patient treated early on, with orally 35 mg of Trimetazidine twice a day, and may suggest his utility in the first line treatment of the phosphine poisoning.
Rare are patients who survive APL poisoning despite intensive medical care. However, our patient survived with no sequelae and remained free of symptoms at six months follow-up because of symptomatic and new supportive treatment.
Aluminium phosphide poisoning is known for its high mortality rates due to severe cardiac toxicity. This may be reversible if the patient can be supported during the initial phase after poisoning.
Prevention remains the best therapeutic way. Regulatory compliance, awareness campaigns by the media and the prohibition of this product ’ s sale to the general public remains mandatory.
No specific drug has been established as an antidote for this poisoning, Hence treatment strategy is mainly supportive until the myocardial injury induced by the active metabolite (phosphine) subsides. Our use of Trimetazidine for this patient with encouraging results needs further validation by others, as it may be a new useful adjunct to aggressive supportive care when needed alongside with other conventional treatments for heart failure. This approach deserves to be studied.
Citation: Minko G, Maaroufi A, Ouazzane R, Azannai I, Zahidi H, Habbal R (2020) Reversible Toxic Myocarditis with Intraventricular Thrombus Associated with Aluminium Phosphide Poisoning Treated with Trimetazidine: A Case Report. J ClinToxicol. 10:444. DOI: 10.35248/2161-0495.20.10.444
Received: 29-May-2020 Accepted: 12-Jun-2020 Published: 19-Jun-2020 , DOI: 10.35248/2161-0495.20.10.444
Copyright: © 2020 Minko G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.