Journal of Cell Science & Therapy
Open Access
ISSN: 2157-7013
ISSN: 2157-7013
Research Article - (2011) Volume 0, Issue 0
The retained activity and Effective half life at 24h of therapeutic 131I in patients with Differentiated Thyroid Cancer (DTC) after total and non-total (sub-total, near-total, partial thyroidectomy and lobectomy) thyroidectomy were compared for patients in these 2 surgical groups. A total of 82 patients (61 females and 21 males) mean age 37.2 ± 9.3 years, mean weight 70 ± 15.6 Kg were considered in this study. 58 patients (70.73%) had papillary Cancer and 24 (29.27%) had follicular cancer. Out of 82 patients, 37 had total thyroidectomy while 45 had non total thyroidectomy (sub-total-29, near total- 10, partial thyroidectomy-4 and lobectomy- 2). 6 patients (7.3%) had metastases. The retained 131I activity (as a percentage of the administered dose in MBq) was 4.61% - 44.56% for patients with total thyroidectomy (mean-26.91 ± 12.57%) compared to 10.18% - 55.36% for patients with non-total thyroidectomy (mean-32.41 ± 12.57%). (p < 0.05) The effective half life ranged between 0.20 – 0.86 days for patients with total thyroidectomy (mean- 0.51 ± 0.21 days) and 0.20 – 1.17 days for patients with non-total thyroidectomy (mean- 0.62 ± 0.27 days). There is no significant difference in the mean effective half lives for the two groups of patients (p = 0.032). Our data suggests that exposure to radiation after ingestion of 131I is similar in both groups studied.
Keywords: Differentiated thyroid cancer; 131I; Total/non-total thyroidectomy; Retained activity; Effective half-life
The role of radioactive Iodine-131 (131I) as a complimentary therapy to surgery in the management of patients with Differentiated Thyroid Cancer (DTC) is well established [1-10]. There is evidence that radioiodine (RAI) therapy is beneficial in terms of prevention of recurrence and lowering of mortality rates in DTC patients [11,12] Doi et al. [12] showed, in their hospital based study, a trend towards improvement in outcome with radioiodine remnant ablation for cancer specific survival. As a result of the benefits of RAI therapy following surgery, it has been considered indispensable in the successful treatment of DTC [13]. The generally accepted indications for 131I therapy according to Freitas et al. [14] are; inoperable primary tumours, post-operative residual tumours in the neck, capsular invasion, local recurrence, presence of distant, cervical or mediastinal metastatic disease.
In spite of the beneficial effects of 131I therapy to patients with DTC, it contributes to the radiation exposure of the population. Some of the organs at high radiation risk are the kidneys and the urinary bladder. This is because RAI is primarily excreted by the kidneys [15]. Radiation dose to the kidneys from RAI therapy, for example, has been found in some patients to be 0.031 ± 0.015 Gy/GBq (mean ± standard deviation) [16] and 0.10 ± 0.06 Gy/GBq for left kidney, 0.095 ± 0.04 Gy/GBq for right kidney respectively [17]. Patients who have renal insufficiency may have impaired RAI clearance which will result in increased retention of iodide in the body [11] with its attendant radiation exposure, hence, diuretics are frequently administered to patients receiving therapeutic RAI so that renal elimination of unbound 131I will be accelerated in order to reduce the adverse effect [18]. It is also said that retention of iodine in the thyroid gland is the result of renal excretion and transport of iodine to thyroid cells [19].
One of the factors that determine the outcome of 131I therapy and retention is radioactive concentration which is the ratio between total uptake and the mass of thyroid tissue in the remnant tissue [11]. The size (mass) of the remnant tissue is not the same for total thyroidectomy and non-total thyroidectomy (near total, sub-total and partial thyroidectomy, lobectomy and isthmusectomy). Even though Total thyroidectomy or near-total thyroidectomy is the preferred type of surgery for patients with DTC and only for small papillary tumours, is more restricted surgery recommended, our surgeons still carry out the other types of surgery mentioned above (as non-total thyroidectomy).
Therefore, it is expected that the concentration of iodide in the remnant tissue and the 131I retention and the effective half life of 131I in DTC patients should be different for the two surgical groups (total thyroidectomy and non-total thyroidectomy respectively). This study is therefore, aimed at surveying the whole body retention of 131I and its effective half life in DTC patients in the two categories with a view to understanding 131I retention and the effective half life of 131I in these patients.
Patients
This study included 82 patients with DTC. 37 patients (45.12%) had total thyroidectomy and 45 (54.88%) non-total thyroidectomy (sub-total-29, near total- 10, partial thyroidectomy-4 and lobectomy- 2). The patients had 131I treatment (dose range 3.36GBq – 12.2GBq mean dose 4.81± 2.02GBq) after an informed consent. 85.2% had initial therapy doses, 11.1% had second dose while 3.7% had a third dose. Patients were treated following induced hypothyroidism with thyroid stimulating hormone (TSH) ≥ 25μIU/ml (optimal level 30μIU/ ml) prior to therapy.
131I capsules administered orally were measured in the dose calibrator (Capintec CRC 15R, Capintec Inc. Pittsburgh, PA U.S.A). The patients were instructed to drink plenty of water to attenuate the radiation dose to the gastric wall before the capsule dissolves. [11] This also helps in attenuating the radiation in the bladder as the patients urinate frequently.
Calculation of retained activity
The measurement of the whole body external exposure rate from the patient at 1m was carried out immediately after ingestion of 131I capsules by the patients [18,20] using a calibrated RADIAGEM 2000 dose rate metre (Canberra Eurisys, Montigny-le-Bretonneux). The retained activity at 24h for each patient was then calculated based on the external exposure rate measurement using
Where A24 is the retained activity at 24h,
A0 is the administered activity,
EWB-24 the whole body external exposure rate at 1m from the patient at 24h EWB-0 is the initial whole body external exposure rate at 1m immediately after ingestion of the 131I capsule.
The measurement and calculations were carried out for each patient until the retained activity reached ≤ 555MBq, the level at which patients are discharged from isolation at our centre. From the calculation of the retained activity, the excreted activity as a percentage of administered activity could also be deduced and the whole body effective half life was calculated as:
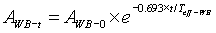 2 [21]
2 [21]
Where AWB-t is the whole body retained activity at t days after administration as measured in equation 1 (in MBq)
WB-0 is the administered activity (in MBq), t is the post administration time (in days)
Teff-WB is the whole body 131I effective half life (in days). Since we considered retained activity at 24h, our t = 1 (in days).
Statistics
Comparison of the mean retained activity and mean whole body effective half lives at 24h post administration between patients with total thyroidectomy and non-total thyroidectomy was obtained using Student’s t-test (Primer of Biostatistics for Windows, version 4.0, McGraw Hill, 1996).
There were 61 females and 21 males (mean age 37.2 ± 9.3 years, range 20 – 58 years). Mean weight of the patients was 70 ± 15.6 Kg (range 48kg – 110kg). 58 patients (70.73%) had papillary Cancer and 24 (29.27%) had follicular cancer. Six patients (7.3%) had metastases. The retained 131I activity (as a percentage of the administered dose in MBq) was 4.61% - 44.56% for patients with total thyroidectomy (mean 26.91 ± 12.57%) compared to 10.18% - 55.36% for patients with nontotal thyroidectomy (mean 32.41 ± 12.57%), P < 0.05 and there was no difference in the pattern of retained activity between those with metastases and those without metastases. Figure 1. shows the chart for the effective half life at 24h for both groups of patients. From the analysis of the data collected for this research, there was no significant difference in the mean activity retained at 24h by DTC patients who had total thyroidectomy and those who had non-total thyroidectomy (p < 0.05) and the mean effective half lives for the two groups of patients were also not significantly different (p = 0.032) even for the patients with metastases compared to those without metastases.
| patient | sex | age | weight | surgery | cancer | status | Te |
|---|---|---|---|---|---|---|---|
| 1 | f | 48 | 94.5 | T | fol | NM | 0.86 |
| 2 | f | 42 | 110 | T | pap | NM | 0.56 |
| 3 | f | 38 | 88.5 | T | pap | NM | 0.42 |
| 4 | f | 39 | 67 | T | pap | NM | 0.23 |
| 5 | f | 32 | 100.2 | T | pap | NM | 0.33 |
| 6 | f | 50 | 80 | T | pap | NM | 0.51 |
| 7 | m | 49 | 64 | T | fol | NM | 0.75 |
| 8 | m | 37 | 57.8 | T | pap | NM | 0.73 |
| 9 | f | 25 | 73 | T | pap | M | 0.39 |
| 10 | f | 34 | 86.5 | T | pap | M | 0.48 |
| 11 | m | 34 | 86.5 | T | fol | NM | 0.81 |
| 12 | f | 23 | 64 | T | fol | NM | 0.51 |
| 13 | m | 20 | 49.5 | T | fol | NM | 0.55 |
| 14 | m | 58 | 54.5 | T | pap | NM | 0.23 |
| 15 | m | 37 | 75 | T | pap | NM | 0.86 |
| 16 | f | 50 | 74 | T | pap | NM | 0.20 |
| 17 | f | 39 | 84 | T | pap | NM | 0.86 |
| 18 | f | 41 | 85 | T | pap | NM | 0.56 |
| 19 | f | 29 | 53 | T | pap | NM | 0.42 |
| 20 | m | 29 | 53 | T | fol | NM | 0.23 |
| 21 | m | 29 | 53 | T | pap | NM | 0.33 |
| 22 | m | 32 | 80 | T | pap | NM | 0.51 |
| 23 | f | 32 | 80 | T | fol | NM | 0.75 |
| 24 | f | 29 | 51 | T | pap | NM | 0.73 |
| 25 | f | 36 | 78 | T | pap | NM | 0.39 |
| 26 | m | 49 | 94.5 | T | fol | NM | 0.48 |
| 27 | f | 43 | 74.3 | T | fol | NM | 0.81 |
| 28 | f | 48 | 94.5 | T | pap | NM | 0.51 |
| 29 | f | 42 | 110 | T | pap | NM | 0.55 |
| 30 | f | 38 | 88.5 | T | fol | NM | 0.23 |
| 31 | f | 39 | 67 | T | pap | NM | 0.86 |
| 32 | f | 32 | 100.2 | T | pap | NM | 0.20 |
| 33 | m | 50 | 82 | T | pap | NM | 0.38 |
| 34 | f | 49 | 64 | T | pap | NM | 0.43 |
| 35 | f | 37 | 57.8 | T | pap | NM | 0.44 |
| 36 | f | 25 | 73 | T | pap | NM | 0.32 |
| 37 | f | 34 | 86.5 | T | fol | NM | 0.41 |
| 38 | f | 34 | 86.5 | ST | pap | NM | 0.75 |
| 39 | m | 23 | 64 | ST | fol | NM | 0.93 |
| 40 | f | 20 | 49.5 | NT | pap | NM | 0.30 |
| 41 | f | 58 | 54.5 | NT | pap | NM | 0.52 |
| 42 | m | 37 | 75 | NT | fol | NM | 0.62 |
| 43 | f | 45 | 74 | NT | pap | NM | 0.49 |
| 44 | f | 39 | 84 | ST | pap | NM | 0.68 |
| 45 | f | 26 | 85 | ST | fol | NM | 0.56 |
| 46 | m | 25 | 53 | NT | fol | NM | 0.96 |
| 47 | f | 29 | 53 | NT | pap | M | 0.63 |
| 48 | f | 29 | 53 | NT | pap | NM | 0.56 |
| 49 | m | 32 | 80 | ST | fol | NM | 0.31 |
| 50 | f | 32 | 80 | NT | pap | NM | 0.52 |
| 51 | f | 29 | 51 | NT | fol | NM | 1.17 |
| 52 | m | 36 | 78 | ST | pap | NM | 0.68 |
| 53 | f | 32 | 94.5 | ST | fol | NM | 0.65 |
| 54 | f | 43 | 74.3 | ST | pap | NM | 0.30 |
| 55 | m | 42 | 57.6 | NT | pap | NM | 1.16 |
| 56 | f | 33 | 88 | ST | pap | NM | 0.23 |
| 57 | f | 46 | 55 | ST | pap | M | 0.75 |
| 58 | m | 58 | 63 | ST | pap | NM | 0.93 |
| 59 | f | 34 | 60 | ST | fol | NM | 0.30 |
| 60 | m | 27 | 61 | ST | pap | M | 0.52 |
| 61 | f | 25 | 52 | ST | pap | NM | 0.62 |
| 62 | f | 30 | 60 | ST | pap | NM | 0.49 |
| 63 | f | 44 | 54 | ST | pap | NM | 0.68 |
| 64 | m | 20 | 61 | ST | pap | NM | 0.56 |
| 65 | f | 42 | 54 | PT | pap | NM | 0.96 |
| 66 | f | 40 | 60 | ST | fol | NM | 0.63 |
| 67 | m | 24 | 58.3 | ST | pap | NM | 0.56 |
| 68 | f | 40 | 85 | PT | pap | M | 0.31 |
| 69 | f | 47 | 66 | ST | fol | NM | 0.52 |
| 70 | f | 46 | 48 | ST | pap | NM | 1.17 |
| 71 | f | 50 | 60 | ST | pap | NM | 0.68 |
| 72 | f | 44 | 72 | PT | pap | NM | 0.65 |
| 73 | f | 31 | 56 | ST | fol | NM | 0.30 |
| 74 | f | 29 | 60 | ST | pap | NM | 1.17 |
| 75 | f | 33 | 55 | PT | pap | NM | 0.23 |
| 76 | f | 41 | 55 | ST | fol | NM | 0.48 |
| 77 | f | 32 | 72 | L | pap | NM | 0.81 |
| 78 | f | 40 | 55 | ST | pap | NM | 0.51 |
| 79 | f | 56 | 54 | L | pap | NM | 0.55 |
| 80 | f | 40 | 58 | ST | pap | NM | 0.23 |
| 81 | f | 50 | 62.5 | ST | fol | NM | 0.86 |
| 82 | f | 39 | 74 | ST | pap | NM | 0.20 |
Key: f=female, m=male, T=total, ST=subtotal, PT=partial thyroidectomy, L=lobectomy fol=follicular, pap=papillary, M=metastasis, NM=No metastasis, Te=effective half life.
Table 1: Patients’ Clinical Data.
Biological half life is said to be reduced by the retention of iodide in DTC patients [11] therefore, it determines the amount of iodide that is retained, since it is related to the elimination of 131I. For most patients it is said that 35% to 75% of administered dose can be expected to be excreted in urine, perspiration and saliva within the first 24h after ingestion [22] and for 131I labelled antibody, the clearance will be about 80% in the first day and the remaining 20% over the next 2 weeks [23]. Our results showed that the excreted activity as a percentage of administered activity ranged from 55.44% to 95.39% (mean 73.09 ± 12.57%) for patients with total thyroidectomy while that for non-total thyroidectomy ranged from 44.64% to 89.82% (mean 67.59 ± 12.57%) which is in agreement with previous works [22,23].
The effective half life of 131I in DTC patients had been calculated for short term (in the first 24h after 131I administration) based on different measuring procedures including external exposure measurement as we did. [21,24,25]. The mean effective half life which was presented from the different groups of authors, based on different number of patients, as presented by Papadimitriou et al. [21] ranged from 0.32d to 7.3d with median values ranging from 0.35d to 0.74d respectively. Papadimitriou et al. [21] and North et al. [25] worked on patients with either total thyroidectomy or near total thyroidectomy while Venencia et al. [24] considered only patients who had total thyroidectomy. In this study we considered the difference between these values for the effective half lives of the two groups of patients. Our values for effective half life for the two groups of patients were in agreement with their own results. Hence, it could be said that for the Nigerian cohort the retained activity and hence, the effective half life of these groups of patients is not different from that elsewhere.
The importance of this study from clinical perspective is based on the fact that in some centres, as we have observed, there are still surgeons who carry out non-total thyroidectomy for patients, even though total thyroidectomy remains the gold standard. In our own experience (in University College Hospital Ibadan), most of the diagnosis of malignant thyroid tissue is done post surgery. In such cases, the surgeon will opt for a partial thyroidectomy in which only the cancerous tissue is removed for histological test with a substantial portion of the healthy tissue left behind with the hope that this will continue to function normally. So when the histological test reveals a papillary or a follicular cancer, the patients will then need a second (completion) thyroid surgery before they could be suitable for 131I ablation therapy [26]. The alternative to the second surgery will be giving of more than one iodine treatment to the patient.
Considering the socio-economic situation in a third world country such as Nigeria, the cost of a second surgery or additional ablative therapy will be very heavy on the patients and this leads to high default in patients’ therapy.
Since effective half life of 131I in DTC patients has been linked to the effectiveness of the radioiodine ablation [11], evaluating the effective half life of iodine-131 in our patients will help us determine how effective these treatments are in the patients. If we know which surgery group has a higher effective half life, we can then say that this group stands a better chance of having a better response to treatment.
The regression and correlation analysis for age versus effective half life as shown in Figure 2 has r and p values of 0.05871 and 0.6003 respectively while that of weight versus effective half life (Figure 3) are r = 0.117 and p = 0.2951. This shows that the outcome of the therapy was not dependent on either age or weight of the patients.
From this study it is concluded that there is no significant difference in the effective half life of 131I in DTC patients in the two categories of surgery.
From the radiation protection point of view, the retention of 131I in DTC patients are similar in both total thyroidectomy patients and non-total thyroidectomy patients which suggests that their exposure to radiation while undergoing ablative therapy is similar. Hence, we suggest that all patients in the two category of surgery be advised to adhere strictly to the same radiation protection measures when undergoing radioiodine therapy.