Journal of Pharmaceutical Care & Health Systems
Open Access
ISSN: 2376-0419
ISSN: 2376-0419
Research Article - (2019)Volume 6, Issue 2
Access to medicines depends on a number of interconnected factors: Medicine prices, one of the most common factors can be affected by the manufacturer’s selling prices, duties, taxes, patent legislation and mark-ups along the supply channel. The South African government has passed price legislation prohibiting discounts and rebates in the pharmaceutical sector by setting a single exit price for all manufacturers and a fee-for service logistics fee for wholesalers, distributors and dispensing fee for retailers as compensation. In this study the South African mark-up structure related to medicine prices is going to be analysed because of its tribulations in implementing a transparent wholesale reform and the fact that it was once classified under the nine countries from the WHO African Region for analysis of data from their Medicines Prices Surveys. This Literature based-analysis study made use of articles, journals for assessing and identifying the denominators responsible for high mark-ups on medications. Documentation pharmaceutical policy papers and publications, researches performed using PubMed and WHO/HAI medicine price database were also used to assess those areas and come up with possible strategies to reduce high price of medication and improve access of healthcare by the public. In private sectors, regulating mark-ups is more complex and weaker than in the public sector which is a reason for higher medicine costs. Policy development need to be reviewed in terms of pricing policies and regulation of mark-ups so that people of South Africa receive the medicine they need at a cost that they and the system as a whole can afford. This structure can help to raise further awareness and prompt the government to evaluate the regulation on entry point drug developers.
Mark-up; Medicine prices; Regulations; Pharmaceutical sectors; Pricing policy; Regulation; Access to medicines; Possible strategies; Supply chain
WHO: World Health Organization; HAI: Health Action International; TRIPS: Trade Related Intellectual Property Rights; MCC: Medicine Control Council; EML: Essential Medicines List; NDP: National Drug Policy; NDH: National Department of Health; GMP: Good Manufacturing Practice; R&D: Research and Development; SADEC: South African Development Community.
In today’s world, the access to medicines depends on a number of interconnected factors of the availability of safety and efficacy, the quality of medicines, its quality assurance, a reliable supply system, rational selection and use of medicines as well as functioning health care system [1]. Even when all those elements are present the lack of affordable medicine prices can prevent access to essential medicines. Medicine prices are usually affected by the manufacturer’s selling prices, duties, taxes and mark-ups along the supply channel. Various retail strategies are often used by suppliers of medicinal products to induce their clients (wholesalers or retailers) to purchase and sell more of their products. These global strategies can include cash rebates, volume discounts, bundling or other deals [2,3]. Each of these can be addressed separately or compositely in attempts to reduce the prices of medicines and therefore facilitate access to essential medicines.
The South African government has passed legislation which makes it unethical for prescribers to be influenced by pharmaceutical representatives in unduly prescribing a product on the promise of an incentive and compel manufacturers to be transparent in their pricing structure. In South Africa, traditional pharmaceutical wholesalers have been a strong force in the pharmaceutical sector. During the 1990’s, pharmaceutical companies started to establish their own distribution channel to supply products whereas others entered into distribution agreements with independent thirdparty logistics companies [1]. These changes allowed manufactures to bypass wholesalers and gain greater control over the supply and marketing of their product. It also increased their margins at the expense of wholesalers [1,2].
In 2004, price regulation was introduced which prohibited discounts and rebates in the pharmaceutical sector setting a single exit price for all manufacturers and a fee-for-service logistics fee for wholesalers, distributors and dispensing fee for retailers as compensation [2,4]. This does however not apply to the national or provincial governments where drugs on the essential medicines list (EML) are acquired through a tender process and supplied to the relevant healthcare facility. Exclusion from this practice is based on the National Department of Health’s aim to provide equal access to medicines for all South Africans through the Essential Drugs Programme.
Another factor that plays a role in medicine price is the patent system which is granted in South Africa to the developers of medicine for legal protection on new and industrially applicable inventions. In this legislation of act 57 of 1978, protection is granted for 20 years from the filing date of the non-provisional patent application. Patent legislation contributes to high prices to cover research and development costs whereas generic drugs are important cost-savers bypassing the developmental stage of drug manufacturing [1,2]. Medicine prices can be considered a crucial element in determining its access by the population, which may be constrained by many factors, both geographic and economic [3]. South Africa was selected for this study as a result of its tribulations in implementing a transparent wholesale reform of the medicine pricing structure and also because it was once classified under the nine countries from the WHO African Region to analyse data from their Medicines Prices Surveys [2,5]. High price mark-up structures could raise further awareness and prompt the government to evaluate the regulation on entry point drug developers [1]. The government subsidies will be taken into consideration knowing that government industries prices should be less than the private companies. This study will help to identify areas in the chain where pricing structures are concerned analysing the critical aspects disabling access to medicines.
Aim
This study aims to identify aspects in the drug manufacturing and supply chain where price increase occurs. The reason for significant differences in the cost of the same drug supplied to both the public and private sector will be investigated.
Objectives
1. Describe, analyze and discuss the mark-up regulations.
2. Identify strategies that can possibly reduce the price of medicines to improve access to quality health care.
This is a literature based-analysis study making use of articles for assessing and identifying the denominators responsible for high mark-ups on medications from the start of manufacturing to the eventual sale to the consumer.
Documentations-pharmaceutical policy papers and publications, E-Drug/E-Med messages- researches performed using Pub Med, and WHO/HAI are used to identify areas where price escalation from mark-ups occurs. The Search engine would be typing ‘pharmaceutical’ AND ‘pricing’ AND ‘south Africa’ yielding to too many hits to be effective and largely identified articles related to pharmaceutical price regulation. The addition or other terms such as ‘mark-up’ OR ‘regulation’ was too restrictive thus resulting to a multiple search approach unless if typed separated along with ‘South Africa’. The search terms included studies in English and French published from 1996 through 2014. Peer-reviewed studies as well as other reports published by government authorities and international agencies were all along with the articles systematically collected through large database searches, filtered and organized into themes. On more than 1000 potentially relevant unique citations, data were obtained from 30 articles, journals or reviews.
This study will be the first of its kind done by the Department of Pharmacology in collaboration with the Department of Financial Governance at the University of South Africa in the contribution to drug regulatory affairs and cost driving factors in drug retail.
Historical overview of pharmaceutical industry
During the 1950’s, multiple tragedies were triggered by unwanted and sometimes disastrous events caused by medications. Examples include the sulfanilamide elixir (presence of highly toxic solvent), vaccine tragedy (diphtheria antitoxin and small pox vaccine contaminations) and thalidomide tragedy (phocomelia). These resulted in substantial increase of legislations for products quality, safety and efficacy as well as stricter norms for Marketing Authorization (MA), Good Manufacturing Practices (GMPs) and most importantly the development of a well-structured drug regulations and control of other drugs. Typically since medicine regulations have been developed after that there has been exponential increase in Information and knowledge on the use of medicines resulting in total Medicines legislation proliferation [6].
Access to medicines
Medicines are described as remedies, drugs, substances or mixed substances used in the treatment, diagnosis and prevention of diseases. Its usefulness is evident on improving abnormal physical or mental state including the promotion of health. The WHO refers to medicines as essential medicines used to satisfy the priority health care needs of the population. They are selected with due regard to disease relevance, evidence on efficacy and safety, and comparative cost-effectiveness [1,7].
The medicine factors that impede medicine prices can be seen as governance weaknesses in pharmaceutical systems. This makes pharmaceutical factors (either a private or public) becoming susceptible to mismanagement and even corruption practices, according to the public. Medicines are well known to promote trust and participation in health services especially when it is meets safety, quality and efficacy. Poor governance availability can therefore increase staff attrition, decrease demand for services, and ultimately reduce program effectiveness. Moreover, lack of access to those essential medicines, their irrational use, and the use of unsafe or poor-quality medicines can surely harm patients [8].
For the use of essential drugs, particularly in primary health care, the development of essential drugs list has been made available across the board which was one of the greatest successes of the authority and Standard Treatment Guidelines (STGs) for the Primary Health Care Level and adult as well as pediatric guidelines for the Hospital Level.
Initiatives, such as the Global Fund to Fight AIDS, Tuberculosis and Malaria, allocate a significant proportion of funding to procure essential medicines and commodities.Others critical weaknesses related to governance and management practices within health systems must come further to light [9].
Interconnected factors governance: WHO is an intergovernmental organization supporting ministry of health concerning development of methods for assessing quality, effectiveness and efficiency.It emphasizes on the importance of governance by identifying it and perhaps is seen as the most critical of its six health system building blocks (accessibility and responsiveness; quality; outcomes; accountability, transparency and regulation; fairness and equity; and efficiency). One of its priorities of work Action Programme on Essential Drugs is to provide technical support to Member States in the development and implementation of national drug policies (NDP) [1,4,10].
NDP steps that closely matched those suggested by the WHO as general advice to all countries are listed as the following:
• More detailed data on price trends in both the private and public sectors
• More analysis of the impacts of policy decisions, with emphasis on indicators of equity, affordability and availability
• Finality on those policy choices which seem to hold clear advantages (such as fixed professional fees and nondiscriminatory exit pricing based on volume)
• Finality on the legal struggle to introduce generic substitution, to regulate marketing practices and to exploit the safeguards provided by the TRIPS Agreement
• Consideration of regional options, including bulk purchasing across the SADC region
The NDP outlines specific health, economic and national development objectives, including the availability and accessibility of essential medicines, the safety and quality of medicines, good dispensing and prescribing practices and individual responsibility for health and informed decision-making [9,11].
The goal of the NDP is to ensure an adequate and reliable supply of safe, cost-effective drugs of acceptable quality to all citizens of South Africa and the rational use of drugs by prescribers, dispensers and consumers while ensuring the availability and accessibility of essential drugs to all citizens [9,11].
1. Importance of good governance: Governance in the Pharmaceutical System is important for relationships between individuals or institutions and the way in which decisions are made and implemented at all levels of the pharmaceuticals [8]. Managing pharmaceuticals in any sector and at any level of the health care system should follow a well-recognized framework. This framework should rely on policies, laws, and regulations, sustaining the commitment to pharmaceutical supply and assuring that good governance principles are followed. Strengthening governance will improves health system performance and ultimately improves health outcomes [8,12].
2. Policies and legislation: These provide framework on how pharmaceuticals sectors should be regulated in a country while controlling the availability, prescribing, and dispensing of medicines as well as the provision of product information. Legislation can include provisions for the licensing and oversight of pharmaceutical establishments and professional staff providing establishment of statutory bodies. Legislation is also needed to regulate clinical trials and to establish mechanisms of post-marketing surveillance to monitor medicine safety. To meet the criteria for good governance, the pharmaceutical policy making process should be participatory and transparent. South Africa definitely needs to strengthen Organizational structures for Appropriate Decision making, Authority, and Oversight [8].
3. Proper program improving access to medicines: This need to be done by increasing transparency and accountability in the health care market place worldwide, to strengthen national capacity to be able to collect, analyze, disseminate, and use data on the very good quality, availability, price, promotion and use of medicines [8].
So, assessing the level of transparency and vulnerability to corruption is the first step in building good governance in the pharmaceutical sector [8]. WHO launched the Good Governance for Medicine programme with the goal of contributing to health systems strengthening and preventing corruption by promoting good governance [10,12].
Quality control and efficacy of medicines
1. Regulatory authorities : The NDP aiming to ensure ‘that drugs reaching patients are safe, effective and meet the approved standards’ relates to the core pharmaceutical aspects of medicine quality, safety and efficacy and falls under the mandate of the Medicines Control Council (MCC) [11,13]. The MCC is responsible for the registration and re-licensing (retention) of medicines, dossier-based medicine evaluations and laboratory-based testing of all medicines used in South Africa in compliance with criteria for medicine evaluation and GMP. MCC serves as an inspectorate of guideline compliance in government depots, hospital stores and private pharmacies and among dispensing health workers on a provincial level, while retaining the specialized functions of inspecting manufacturing facilities and wholesale premises at a national level [11,13,14].
2. Quality assurance requirements: Pharmaceutical and analytical quality assurance requirements of the MCC encompass the pharmaceutical and biological availability, details on the active pharmaceutical ingredient, the formulation, specifications and control procedures for pharmaceutical ingredients, containers and packaging materials, manufacturing procedures, stability data of the finished pharmaceutical product, pharmaceutical development and the expertise and premises used for the manufacture of a biological medicine. If all of those are not met by the applicant, the medicine will not be registered and thus not being available to the public [9,13].
3. Prescribing and dispensing: The NDP development of ‘human resources to promote the concepts of rational drug use’ is enabled by pharmaceutical support staff appointed for an optimal distribution chain. Multidisciplinary hospitals are recommended in the public and private sector to ensure efficient and cost-effective medicine supply and use by compilation of a hospital formulary and good supply chain management. According to the law and regulation it is only licensed practitioners who may prescribe and/or dispense medication [1,9,13].
A three-tiered demand structure exists-with the prescribers as the actual demanders, secondly patients as the consumers, and thirdly the healthcare system consisting of the public and private sectors. Consumers of drugs or patients are mostly dependent on a prescriber. They are not fully responsible for the choices made; the prescriber is the one who legally determines the need and therefore enabling access to drug. Thus this may create a problem for the consumers who lack of necessary funds to provide themselves the authorized person for prescription note to buy the medicine they need [4,15].
Patent legislation: The patent law of the intellectual property system is granted in South Africa as in the case of many other countries, providing the legal protection granted for 20 years of a new and industrially applicable invention [16,17]. The South African patent act no 57 of 1978 was amended up to act no 49 of 1996. In this system, the invention can be a product or process considered new based on invention step in the same idea which has not been expressed elsewhere immediately prior to the date of the invention [17]. In term of medicines, this is the procurement of originator brand used by manufacturers, impeding access to medicine because of the protection which results in the price of medicine to be expensive [16]. Furthermore, the Patent cost is more expensive in South Africa as compared to other countries such as India of which is the majority of our medicines originated from. That is why a range of policy is now available to promote generics medicines which are important cost- savers [17,18]. High research and development (R&D) costs are often assumed to be responsible for the high costs of innovator/originator drugs [4,16].
Pharmaceutical sectors: Pharmaceutical sectors play a role in the price of medicines. In the private sector, drugs are one of the major cost drivers in healthcare expenditure culminating in a highpremium, unaffordable luxury to the general population. The high price of medicines, which generate large profits, is the result of pharmaceutical companies’ discretionary price increases which is not necessarily linked to inflation. Private sector healthcare systems tend to lack published data available for evaluation of their performance in the pharmaceutical industry according to Basu et al. [19]. Most pharmacists are found in this sector with all registered drugs are available, but only serving 20% of the population [1,2]. Contrary to prevailing assumptions, the private sector appears to have lower efficiency than the public sector, resulting from higher drug costs, perverse incentives for unnecessary testing and treatment and weak regulation [19].
In the public sector the cost to provide health care services, drug costs are second only to human resource related expenditure. The public sector is mainly supplying essential drugs as specified in the NDP and Treatment guidelines and is supplied free of charge to specific groups of people, providing more equitable and evidence-based care [2,19]. The total cost relating to public health care amounts to 11% of government budget and is linked to a controlled inflation rate. Less than 50% of registered pharmacists in South Africa are employed in the public sector, which serves the majority of the South African population [2].
High price of medicine particularly in the private sector is a key barrier to affordability of essential medicine while affordable medicine prices are the key determinant in improving access to healthcare [20].
Furthermore, it is known that the medicine costs do not even include the other costs such as consultation and diagnostic tests. Patients who cannot afford the private-sector costs may do without medicines completely. These findings are in line with other studies of affordability of chronic medicines which indicate that these medicines are unfortunately unaffordable for many populations [21-24].
Supply and distribution chain and management for human health: Traditional supply chain is a system of all parties that are directly or indirectly involved in fulfilling the patient or consumer request for medicines. ‘The supply chain is often, although not always, organized separately and operated independently between the private and public health sectors’. In this ‘Figure 1’ below which is the case of South Africa, the manufacturer or the importer of drugs sells in bulk to the wholesaler or distributor at a single price irrespective of volumes, number rebates, discounts or any other controversial practice. In the case where the manufacturer is present outside the country of sale, there may be an importer or trader acting between the manufacturer and wholesaler, although in some cases the wholesalers do the importation themselves [1].
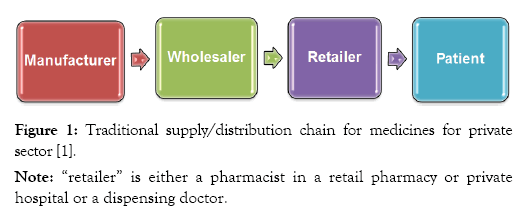
Figure 1: Traditional supply/distribution chain for medicines for private sector [1].
The wholesaler then sells smaller quantities to retailers who are usually a private or public pharmacy but could be general traders, dispensing doctors, or other authorized selling points for medicines-it is at this point where mark-up starts. The retailers finally sell the products to the consumers. Normally retailers cannot keep everything in stock due to limited space or because storage demands or special requirements [1,16].
Private sector facilities can purchase medicines directly from wholesalers and pharmaceutical companies, all of which must be approved by the MCC [1].
There are numerous of different supply chain models that are in operation in the public sector in various countries (in ‘Figure 2’ above). As with the private sector model, the manufacturer may be domestic or international, and may supply through intermediaries such as international procurement agents. Depending on the size of the country and the distribution model, there may be a greater or lesser need for lower level stores [1].
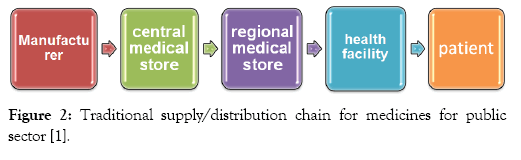
Figure 2: Traditional supply/distribution chain for medicines for public sector [1].
Medicine pricing
What is a single exit price: It is the price set by the manufacturer or importer of a medicine or Scheduled substance in terms of regulations combined with the logistics fee and VAT and is the price of the lowest unit of the medicine or Scheduled substance within a pack multiplied by the number of units in the pack [1,25]. Furthermore the ‘single exit price’ which is a flat fee system for dispensers, has replaced the mark-up system. Under the law, the dispensing fees for prescription drugs were set at a maximum 26% of the manufacturer’s selling price. After repeated disputes and court matters [1], the government revealed the new pharmacy dispensing fee system where we find a series of mark-up percentage ceilings within medicine price brackets. Under the new structure, the pharmacies are seen to charge less for low-priced, high-volume medicines such as antibiotics, but can increase their fees for higherpriced products. Although it was hoped that this progressive system would increase access among low income patients, at the expense of independent, small pharmacies that are unable to sustain falling profit margins [9,13].
Dispensers may charge an additional dispensing fee depending on the price of the medicine. The Medicines and Related Substances Act allows for the following charges (excl VAT) [26]:
• Where the SEP is less than R85.70, the maximum dispensing fee is R6.95 + 46% of the SEP.
• Where the SEP is less than R228.53, the maximum dispensing fee is R18.55 + 33% of the SEP.
• Where the SEP is less than R799.85, the maximum dispensing fee is R59.00 + 15% of the SEP.
• Where the SEP is greater than or equal to R756.00, the maximum dispensing fee is R140.00 + 5% of the SEP.
According to the code of South Africa, prices listed in this database represent the maximum price that you should be paying for your medicines [26].
Drug prices are therefore considered to a large extent to be managed by their manufacturers first, rather than by the market. They have more to do with the manufacturing and development costs of the particular product, and less to do with the characteristics of the market in which they are placed (including average incomes, types of social security, exchange rate fluctuations, competitor price levels and future research and development costs) [9,26].
Specific cost containment measures that were signaled in 1996 were [4]:
• A pricing committee, to “monitor and regulate drug prices”
• Total transparency in the pricing structure (at all points of the distribution chain)
• A non-discriminatory pricing system
• Replacing the wholesale and retail mark-up system with one based on a fixed professional fee
• A database to monitor costs compared with other developing and developed countries
• Regulation of price increases
• Provision, in certain circumstances, of public sector stock to the private sector (e.g. supplying lower cost drugs bought by the State to private sector clinics in order to address a priority disease)
• Promotion of generics (multi-source pharmaceutical products, generally cheaper than the originator’s branded products), including generic substitution, while maintaining a negative list (a list of drugs that could not be substituted by the pharmacist at the patient’s request, but where the prescribed brand would have to be supplied)
• Measures to improve rational drug use, including establishing Pharmacy and Therapeutics Committees (PTCs) in all hospitals
• Control of pharmaceutical marketing practices generic substitution and parallel importation remain hostage to that court action.
The rational use of drugs aim to promote the rational prescribing, dispensing and use of drugs by medical, paramedical and pharmaceutical personnel and to support the informed and appropriate use of drugs by the community. Price regulation was introduced prohibiting discounts and rebates in the pharmaceutical sector setting a single exit price for all manufacturers and a fee-forservice logistics fee for wholesalers, distributors and dispensing fee for retailers as compensation [2,4,11].
Internal reference pricing systems involve a national authority setting local prices for a drug by comparison with similar drugs on the national market (e.g. deciding that a new anti-hypertensive drug will be similar in price to other drugs already available to treat hypertension [4].
Mark-up regulation
What is a mark-up: It is defined as the difference between the purchase price or cost price of a product and retail price of that same product, thus representing all the additional charges and costs applied on a product in order to cover overhead costs and distribution charges to generate a profit [1]. It may also be described as the “gross profit” of a product. A Mark-up that is incurred within a distribution cycle can be influenced by commercial practices involving discounts, rebates and other trade schemes which leads to the increase of medicine prices [1,2].
It is possible to introduce flexibilities in mark-up regulations making allowances for the type of product and/or its price or other variables. Therefore possible to have separate strategies for originator brand and generic medicines so on the national essential medicines list and those not on the list, or have some form of regressive mark-ups whereby higher cost products receive lower mark-ups and thus create or influence incentives within the supply chain. Regressive mark-ups can have a differential impact on originator brand and generic medicines where these are marketed at different prices [1,4].
One of the ministries of health issue was to replace the mark-up on drugs by a professional fee for the pharmacist because part of the problem was the current huge mark up. Considering that there should be one price to be transparent to know what the mark up is between the exit price from the manufacturer and the price that is paid by the patient [9] as mentioned previously.
Another priority obviously was to encourage generic prescribing and generic substitution because people did not even know that for the same illness it is possible to prescribe the same pharmacological substance but under different brand names and very different prices [9].
Generic substitution policy is one strategy that needs to be followed to bring down medicine prices even though it somehow stimulates competition among manufacturers. Nowadays, the buyers of products still pay an unreasonable price for certain medications which are already available in the generic form without knowing. The buyers can pay a reasonable price for the same product imported from elsewhere. So we need to be able to import drugs if we can’t get them reasonably priced in South Africa. This action is known as parallel imports [4,5,9,13].
What is parallel importation: Parallel importation involves the purchase from a supplier abroad of a product already on the local market, manufactured by the same company but sold at a higher price. International tendering involves the purchasing on a competitive basis from manufacturers or other suppliers in countries outside South Africa [4,20].
The pharmaceutical industry of South Africa is relatively well developed and mostly focused on the production of generics, including manufacturing copy medicines under license [20].
This should continue to allow better access of medicines to patient. Possible strategies
A five-point plan envisaged, looking at drug cost structures in the public sector, the development of an essential drugs list, the promotion of generic drug use, a review of the regulatory authority and incorporation of traditional medicines should be implemented [27].
The ministry of health needs to review incomplete regulations constraining drug pricing and legislations and encourage South African Drug Action Programme establishment playing a major advocacy role dealing with any issues that arise from the implementation of the drug policy needing clarification and attention [8,27]. This Programme also provides feedback on what’s happening at the grassroots level. So its role is be not only to facilitate the implementation of the policy but also to monitor what’s going on in terms of results, opinions and actions of stakeholder [27].
The authority needs to set up good distribution chains with good Management skills.
Health workers needs to be able to predict when stock will be used up more quickly and an urgent order, this can be fixed though the use of Human resources development aiming to develop expertise and human resources to support the implementation of the policy and to promote the concepts of essential drugs and rational drug use and ensure their adoption throughout the country. This will help to make health care more accessible [28-30].
The purchase of medicine from manufacturer is the key factor that influences access. This can be best addressed though price regulatory system. South Africa needs a simplified harmonized and efficient transparent regulatory approval process as medicine generics, which is well implemented in an integrated manner [5,29].
The ministry of health and other authorities should continue to support with the campaign development such as the marching to development of trade and industry (DTI), the treatment action campaigns (TAC), doctors without borders (MSF) and SECTION 27 who demand finalization of a national Intellectual Property Policy to provide South Africa with better access to medicines required through legislative reform [18].
Increase consumer awareness and acceptance of good quality generic equivalents. Awareness creation and promotion of generic acceptance in the community and amongst healthcare professionals should be done [28] E.g. when prescription is made out of a brand product by doctors, pharmacists should propose patients on the possibility to have the same drug but as a generic in a cheaper price [26].
Removal of all taxes and tariffs including VAT on medicines, especially essential medicines e.g. the authority should also consider the fact that Medicine prices should be managed in terms of the economic standard of the population for a better access to medicines. Because of high percentage of poverty and unemployment, South Africa is known to have a small wealthy population and medium sized middle income [1,5].
Innovator drugs are usually not well covered by reference price systems, as no comparator products exist. This side of the pricing committee should also be reviewed [4].
Information obtained through literature review is public knowledge, but no specific pharmaceutical manufacturer will be named or identified in order to prevent unfair bias.
Regulation of mark-ups as part of a comprehensive price regulation strategy probably will lead to reduced medicine prices. However, regulation of mark-ups without regulation of either the manufacturer’s selling price or the retail selling price is unlikely to lead to reduced medicine prices. In private companies regulating mark-ups is probably more complex than in the public sector. While banning discounts rebates and bonuses in the supply chain probably increased transparency in medicine pricing but there is insufficient evidence to say whether it leads to reduced price. The real test is in implementation of work policy to satisfy the public which should be supported by good governance.
The public will only be happy when prices start to go down because nowadays, people want visible action and results to follow statements of good intent. This commitment must go beyond lip service to include active participation in the process of initiation, review and modification to ensure that the people of South Africa receive the drugs they need at a cost that they and the system as a whole can afford.
The poor governance is costly for governments and result not only to valuable health sector resources lost, consumers or buyers of products un-satisfaction but also in future donor funding compromised due to the loss of credibility affecting the economic sector. The high market value of medicines makes them a target for theft according to consumers.
Policy development need to be reviewed in terms of pricing policies and regulation of mark-ups that are required to increase availability, reduce prices, and improve affordability and also include more sectors and other essential medicines. It should contain aspects of price control and incentives in order to reduce prices.
Further research to find out factors that impact on medicine prices within private and public sectors by comparison to medicine prices making use of a survey for assessing and identifying the denominators responsible for high mark-ups on a particular medication from the start of manufacturing to the eventual sale to the consumer would be one of my further objective.
Many thank with gratitude to the contributions of the following individuals and organizations. WHO/HAI Pricing Policy Working Group for providing guidance and reviewing the paper, to the NDP and NDH Member of States for the development and implementation of pricing committee, to the University of Pretoria department of Pharmacology who thought us better understanding or Regulatory Pharmacology, with special thanks to my supervisor Dr. A Marais for all his support and guidance throughout my research as well as Mr. A Gray for assistance on main regulations affecting pricing in South Africa.
Citation: Ondo ZG (2019) Regulatory Analysis of Mark-up Structure in Medicine Prices by the Pharmaceutical Industry in South Africa. J Pharma Care Health Sys 6: 203. doi:10.35248/2376-0419.19.6.203
Received: 02-Jun-2018 Accepted: 15-Mar-2019 Published: 22-Mar-2019 , DOI: 10.35248/2376-0419.19.6.203
Copyright: © 2019 Ondo ZG. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.