Lupus: Open Access
Open Access
ISSN: 2684-1630
ISSN: 2684-1630
Mini Review - (2025)Volume 10, Issue 1
Systemic Lupus Erythematosus (SLE) pathogenesis includes a compromised clearance of nucleic acids, increased type I Interferon (IFN) response, dysregulated B cell tolerance causing an increased autoantibody synthesis, immune complex formation and deposition. Autoantigens released because of the defects in regulated cell death pathways could be presented by follicular dendritic cells to autoreactive B cells in Germinal Centers (GCs) resulting in loss of self-tolerance causing production of autoantibodies, immune complexes, and pro-inflammatory cytokines leading to inflammation and tissue damage in SLE. In numerous pathological states in vivo, cells exhibit a variety of active, programmed patterns of cellular necrosis, such as apoptosis, necroptosis, pyroptosis, NETosis, autophagy and ferroptosis. These necrotic cell deaths are generally characterized by cell swelling, rupture of the cell membrane and release of the cell's contents into the surrounding tissues. This review delves into different dysfunction of Regulated Cell Death (RCD), elucidating their roles in SLE progression and offering insights into novel therapeutic avenues.
Systemic lupus erythematosus; Regulated cell death; Autoimmunity; Therapeutic targets; Immune dysregulation; Innovative therapies
How might unraveling the intricacies of regulated cell death pathways transform our approach to treating Systemic Lupus Erythematosus (SLE) where traditional therapies often fall short?
Complexity in the clinical features of SLE indicates presence of several subsets of SLE, with an underlying unique combination of disease pathways, genes and environmental factors. Traditional SLE treatments are broadly based on NSAIDs, corticosteroids, antimalarial drugs, immunosuppressants and biologics, offering limited specificity. Regulated Cell Death (RCD), a genetically guided process, eliminates infected, damaged or diseased cells, releasing intracellular components that trigger proinflammatory gene expression in immune cells. Excessive debris externalization activates the immune system, fostering antigen-antibody immune complex formation across various tissues [1]. In multicellular organisms, genetically RCDs including apoptosis, necroptosis, pyroptosis, NETosis, autophagy and ferroptosis were considered an important pillar for maintaining homeostasis. Endogenous selfantigen and nucleosomes from apoptosis and defective clearance cells in SLE will activate plasmacytoid Dendritic Cells (pDCs), through the Toll-Like Receptor (TLR) 7 and TLR 9 to interact with pathogenic self-reactive T cells for type I Interferon (IFN) production, and with B cells for the manufacture of autoantibodies [2]. These self-immune complexes also can cause the process of Neutrophil Extracellular Traps (NETs) release (NETosis), the fibrous networks protruding from activated neutrophils in response to infection or inflammation [3]. Through downregulated expression of Glutathione peroxidase 4 (GPX4) and elevated lipid Reactive Oxygen Species (ROS) levels, neutrophil ferroptosis also leads to stimulation of autoreactive B cells and pDCs, autoantibody and IFN production, finally contributing to disease manifestations [4]. Necroptosis is an alternative pathway when the apoptosis pathway is inhibited. Elevated constitutive IFN signaling in SLE also contributes to the steady-state expression of MLKL and the augments of necroptosis [5]. RIPK3 that is essential for necroptotic signaling can promote the NLRP3 inflammasome hyperactivation, pyroptosis responses and eventual IL-1β and IL-18 inflammatory in patients with SLE and Lupus Nephritis (LN) [6]. Molecular Chaperone-Mediated Autophagy (CMA) in B cells and abnormal immune type I IFN secretion by macrophages are involved in the pathogenesis of SLE. High expression of IFN-Α inhibits mTORC1 and activates reactive oxygen species to induce autophagy in podocytes [7]. The convergence of precision medicine and pioneering strategies centered on these intricate pathways has the potential to expand the landscape of SLE treatment.
Molecular interaction pathways of RCDs
In response to DNA damage, oxidative stress or nutrient deprivation, both transcriptional and post-translational activation of the pro-poptotic BCL-2 family are critical in transducing the pro-apoptotic signal to drive mitochondrial outer membrane permeabilization. In mice, transgenic overexpression of BCL-2 in lymphocytes causes a fatal Systemic Lupus Erythematosus (SLE)-like autoimmune disease and a low incidence of lymphoma. Moreover, extrinsic apoptosis is mediated by the activation of intracellular protein-protein interaction domain known as the Death Domain (DD), such as Fas (also known as CD95), TNFR1, TRAIL (DR4) and TRAILR2 (DR5) [8].
Genetic defects in Fas and its ligand FasL lead to a rare human autoimmune condition known as autoimmune lymphoproliferative syndrome, which can be phenocopied by Fas and FasL mutant mice [9]. Caspase-8 activation plays an important role in Fasmediated apoptosis. Phagocytosis is important for the noninflammatory nature of apoptosis. If apoptotic cells fail to be “eaten,” they can eventually develop membrane damage. The release of intracellular Damage-Associated Molecular Patterns (DAMPs) sounds the “alarm” to neighboring cells and triggers a pro-inflammatory response. DAMPs include DNA, ATP and proteins such as High Mobility Group Box 1 (HMGB1) and IL-1α [10].
If active RIPK1 is not cleaved by caspase-8 in death-inducing complex II, then it can engage the kinase RIPK3 to unleash caspase-independent Mixed Lineage Kinase Domain Like (MLKL)- mediated necroptosis [11]. RIPK1 autophosphorylation following DD-mediated dimerization of the kinase80 licenses RIPK1 to enter a secondary, cytoplasmic death-inducing complex II [12].
Caspase-1, -4, and -5 in humans and caspase-1 and -11 in mice, each cause pyroptosis by cleaving Gasdermin D (GSDMD). Caspase-1 forms active dimers within cytoplasmic complexes called inflammasomes [13]. The pyroptotic cells also release mature IL-1Β because GSDMD cleavage elicits perturbations that activate the NLRP3 inflammasome and caspase-1 [14].
Necroptosis also can trigger NLRP3 inflammasome activation within the dying cell, resulting in activation of caspase-1 and the release of active IL-1Β [15].
One of the pathogenic mechanisms of aberrant neutrophil activation in SLE is the formation of Neutrophil Extracellular Traps (NETs), web-like structures constituted of cytoplasmic and granule components with nucleic acids from nucleus or mitochondrial [16]. Downstream signaling pathways in neutrophils, such as ROS, either from NADPH oxidases or mitochondria, promote chromatin decondensation by stimulating Myeloperoxidase (MPO), neutrophil elastase and Protein-Arginine Deiminase type 4 (PAD4) [17]. Phorbol Myristate Acetate (PMA) can induce NETs dependent on ROS production by NADPH oxidase. PMA also can induce autophagic vacuolization, which is independent of Nox2 activity. Of interest, blocking Nox2 or autophagy can inhibit NETosis, resulting in a cell death characterized by features of apoptosis, suggesting that when autophagy or Nox2 activity is restrained, apoptosis may serve as a backup type of death for NETosis [18].
Activation of autophagy in necroptosis promotes the degradation of cellular debris. Autophagic cell delivers cytoplasmic contents to the lysosome for degradation and is important in promoting cell survival under stress [19]. Lysosomal cell death is executed by cathepsin-mediated cleavage and activation of key regulators of apoptosis, such as caspases and BCL-2 family members, as well as by the direct action of cathepsins on key pro-survival mechanisms [20].
Ferroptosis, refers to the accumulation of lethal iron-dependent phospholipid peroxides on cell membranes when Glutathione Peroxidase 4 (GPX4) is inhibited, leading to non-apoptotic cell death.
How RCDs are involved in the immune response and leading to innovative SLE therapies?
In mice, transgenic over-expression of BCL-2 in lymphocytes causes a fatal Systemic Lupus Erythematosus (SLE)-like autoimmune disease and a low incidence of lymphoma. Enforced expression of BCL-2 suppresses intrinsic apoptosis and can cause human follicular lymphoma. Preclinical studies have shown that APG-2575, a selective Bcl-2 small molecule inhibitor, competitively binds to Bcl-2 proteins and induces apoptosis of overpopulated T cells, B cells and other lymphocytes in a model of systemic lupus erythematosus. Inhibiting the differentiation and survival of Th17 cells in SLE patients is known to attenuate inflammatory damage and delay disease progression. Apoptotic Vesicles (apoVs) are metabolic Extracellular Vesicles (EVs) released from apoptotic cells. Apoptotic cells and apoVs generated H2S inhibiting aberrant Th17 cell differentiation in MRL/lpr mice via sulfhydration of Sep15. A correlation between downregulated serum exosomal miR-451a expression and SLE disease activity, renal damage as well as lymphocyte communication. In identifying active renal disease in SLE patients, Soliman et al. found urine/serum fractional excretion ratios are better than the corresponding urinary biomarker proteins. Targeting caspases has not proven a fruitful therapeutic strategy to date, with inhibitors exhibiting limited efficacy as well as toxicity in clinical trials.
Cutaneous lupus erythematosus patients are being trialed with the RIPK1 inhibitor. The pro-inflammatory nature of pyroptosis is a boon in combating pathogens because immune cells, including neutrophils, are recruited to the site of infection. The downside is that excessive pyroptosis from dysregulation of the pathway can cause disease. GSDMD pores permit DNA release from lupus neutrophils.
In SLE, neutrophil mtDNA is highly accessible to be oxidized by mitochondrial Reactive Oxygen Species (mROS) and released from mitochondria. Cytosolic mtDNA further promotes IFN production through the cGAS-STING pathway. In addition, mtDNA can also be released into the extracellular environment. Extracellular mtDNA activates plasmacytoid dendritic cells and initiates CD4+ T cell activation, which is critical to SLE pathogenesis. Self-DNA complexes derived from NETs promote the differentiation of IgG2-secreting B cells and activation of B cells via TLR9 activation, thereby promoting the proliferation of autoreactive memory B cells and the production of autoantibodies. NETs, through Matrix Metalloproteinases (MMP) activation, lead to endothelial cell apoptosis and disruption of endothelial integrity, promote type I IFN production and promote inflammation. Defects in macroautophagy/autophagy contributed to the pathogenesis of SLE, especially in adaptive immunity. Li et al., revealed markedly increased levels of both autophagy activation and NET formation in neutrophils from SLE patients [4]. Hydroxychloroquine can suppress NET formation by inhibiting autophagy within a PMA-induced NETs system.
Ferroptosis has been implicated in many diseases, but it is difficult to test causality in pre-clinical disease models genetically because mediators of ferroptosis also have critical roles in normal cell metabolism. Detection of ferroptosis is another challenge because there is no one discriminating marker unique to this form of cell death. Lipid peroxidation must be observed, and a combination of markers evaluated to exclude other forms of cellular stress. Pristane (2,6,10,14-tetramethylpentadecane) is a C19 isoalkane used as an inflammation inducing agent causing lupus, arthritis and myeloma development in several rodents and Pristane-Induced Lupus (PIL) is used as a classical animal model of SLE. Gao et al., have used PIL to study how iron insufficiency promoted expansion of Treg cells by reducing ROS production and improved the clinical disease.
Dysregulated cell death pathways and pathogenic mechanisms in Systemic Lupus Erythematosus (SLE). (A) Apoptosis perturbation. The extrinsic apoptosis pathway initiates upon death receptor binding, triggering a caspase cascade, driving the outer membrane permeabilization (MOMP) and commits cells to death. (B) Aberrant NETosis eventually lead to cell membrane rupture and NETsrelease. (C) Various cell types that are involved in the development of SLE. (D) Ferroptosis dysregulation. TFR1 facilitates ferritin transportation to the cell membrane. Elevated free iron and peroxides trigger the Fenton reaction, ultimately driving ferroptosis. (E) Autophagic abnormality. These autophagosomes then fuse with lysosomes, leading to the breakdown of the enclosed contents by lysosomal enzymes. (F) Necroptosis disturbance. The necrosome, stabilized by RIPK1, RIPK3 and autophosphorylated MLKL, prompts phosphorylated MLKL binding to the cell membrane. This process disrupts cell integrity, exposing danger signals (DAMPs). (G) Necroptosis disturbance. The NLRP3 inflammasome activates caspase-1, which activates GSDMD, the precursor of Pro-IL-1β and Pro-IL-18, respectively. Subsequent oligomerization of GSDMD-NT forms transmembrane pores in the plasma membrane, resulting in the release of IL-1β and IL-18. (H) Regulated Cell Death (RCD) is induced by the triggering agent through cascades of cellular signaling and inflammatory responses. The apoptotic autoantigens released during the process activate Antigen-Presenting Cells (APCs), promoting tissue damage. Activated APC causes T cells to produce cytokines and B cells to produce other antibodies and complexes, resulting in tissue damage (Figure 1).
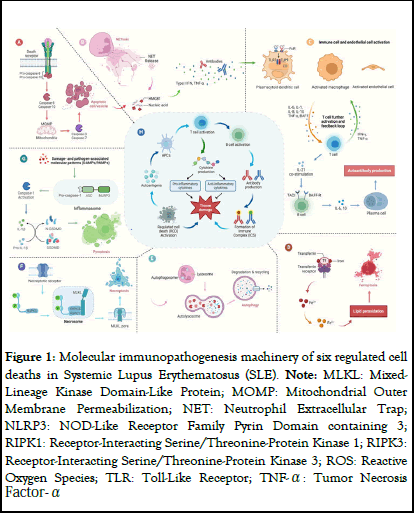
Figure 1: Molecular immunopathogenesis machinery of six regulated cell deaths in Systemic Lupus Erythematosus (SLE). Note: MLKL: Mixed- Lineage Kinase Domain-Like Protein; MOMP: Mitochondrial Outer Membrane Permeabilization; NET: Neutrophil Extracellular Trap; NLRP3: NOD-Like Receptor Family Pyrin Domain containing 3; RIPK1: Receptor-Interacting Serine/Threonine-Protein Kinase 1; RIPK3: Receptor-Interacting Serine/Threonine-Protein Kinase 3; ROS: Reactive Oxygen Species; TLR: Toll-Like Receptor; TNF-α: Tumor Necrosis Factor- Α
The critical step of the intrinsic apoptosis is the activation of the pro-apoptotic effectors of the BCL2 family, BAX, BAK and possibly BOK, which drives the outer membrane permeabilization (MOMP) and commits cells to death. MOMP results in the release from the mitochondrial intermembrane space into the cytosol of proapoptotic proteins. Although apoptotic cells themselves induce immune tolerance by inducing anti-inflammatory cytokine production, secondary necrosis occurs when apoptotic cells are not cleared in a timely and effective manner, which can significantly promote inflammatory factor release, enhance the immune response and participate in the pathogenesis of SLE. Therefore, preventing the secondary necrosis of apoptotic cells and maintaining cells in the apoptotic state may be one strategy to prevent autoimmune diseases such as SLE. The secondary necrosis after apoptosis was also a regulated process called pyroptosis that was mediated by Gasdermin E (GSDME). GSDME has roles in switching the cell death mode from apoptosis to pyroptosis. In GSDME-high cells, the previously presumed key apoptosis inducer, activated caspase-3, cleaves GSDME to generate the GSDME-N domain. Subsequently, the GSDME-N domain assembles in the cell membrane to form pores, thereby resulting in secondary necrosis. The inhibition of these pathways, as explored in various studies, offers a strategy to mitigate the inflammatory response, albeit with the challenge of avoiding impairment of pathogen defense mechanisms. For instance, RIPK1 inhibitors' role in curtailing necroptotic signaling provides an illustrative example of targeted intervention that could mitigate inflammatory damage without compromising cellular defenses. In SLE, the level of neutrophil ROS is significantly increased, leading to NETs produced in large quantities, which results in the release of excessive inflammatory factors and aggravating the inflammatory response. NETs have also been found to be a source of various autoantibodies and are the initiating factors leading to the development of SLE. So too does the potential to target this pathway to ameliorate disease manifestations associated with oxidative stress and lipid peroxidation. As our understanding of this iron-dependent form of cell death expands, so too does the potential to target this pathway to ameliorate disease manifestations associated with oxidative stress and lipid peroxidation. Importantly, the translation of these insights into clinical practice necessitates a nuanced approach, given the pleiotropic roles of these cell death pathways in maintaining physiological homeostasis and responding to environmental insults. Precision medicine, informed by the molecular and cellular underpinnings of SLE, promises a more individualized and effective therapeutic strategy, potentially overcoming the limitations of current broad-spectrum immunosuppressive and anti-inflammatory treatments.
Cell death is required for the survival and fitness of multicellular organisms but must be tightly regulated to prevent disease. A comprehensive understanding of cell-death signaling mechanisms has revealed opportunities to counter aberrant cell death in disease. Effectors of pyroptosis, such as NLRP3 and GSDMD, are targets of interest, along with RIPK1 as a mediator of extrinsic apoptosis and necroptosis. Key to these efforts knows when and where the different cell-death pathways are activated in human disease. Molecular markers of the various forms of cell death have been defined, for example, phosphorylated RIPK3 or phosphorylated MLKL for necroptosis and cleaved GSDMD for inflammasome-induced pyroptosis, but their presence in tissues is likely ephemeral owing to the rapid clearance of dead cells by phagocytes. Therefore, detection of pathway activation markers can be challenging even in pre-clinical models where the genetics tell us the pathway is active. What really matters is whether interruption of a death pathway will result in clinical benefit.
With a deep comprehension of these diverse mechanisms, therapeutic avenues explored in this review have the potential to untangle the complexities of SLE.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Tan Q, Huang W, Zheng Y, Wen Y, Li M, Tao Y, et al. (2025) Regulated Cell Death in Systemic Lupus Erythematosus: Dysfunction of Cell Metabolism. Lupus: Open Access. 10:336.
Received: 29-Mar-2024, Manuscript No. LOA-24-30550; Editor assigned: 01-Apr-2024, Pre QC No. LOA-24-30550 (PQ); Reviewed: 15-Apr-2024, QC No. LOA-24-30550; Revised: 08-Jan-2025, Manuscript No. LOA-24-30550 (R); Published: 15-Jan-2025 , DOI: 10.35248/2684-1630.25.10.336
Copyright: © 2025 Tan Q, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.