
Biochemistry & Pharmacology: Open Access
Open Access
ISSN: 2167-0501

ISSN: 2167-0501
Research Article - (2018) Volume 7, Issue 2
Background: Pomegranate (Punica granatum) of the Punicaceae family is an ancient fruit which is rich in nutrients and also contains various bioactive compounds adduced to some of its medicinal properties observed in vivo.
Aim: The aim was to determine the proximate, phytochemical composition and the antioxidant activity of pomegranate seed extract in female albino rats.
Methodology: Crude extraction protocol was done using ethanol. The proximate and phytochemical composition of the seeds was determined using standard methods. Sixteen rats were divided into four groups. Rats in group I served as control, while rats in group II, III and IV received 200 mg/kg, 300 mg/kg, and 400 mg/kg per body weight of P. granatum seeds extract orally respectively for 14 days. Glutathione (GSH), Catalase (CAT) and Superoxide (SOD) levels were determined from the serum using standard kits.
Result: Proximate analysis shows the contents of moisture (6.84%), ash (1.55%), fiber (27.59%), protein (14.06%), fat (26.03%) and carbohydrate (23.96%). Phytochemical screening reveals the contents of flavonoids (121.22 mg/g), tannins (3.30 mg/g), saponins (12.87 mg/g), alkaloids (1.51 mg/g) and phenols (78.12 mg/g). There was a significant (p<0.05) increase in the GSH, CAT and SOD levels of the groups that received the extract, while the highest significance was noted with the group that received the highest dose as compared to the control.
Conclusion: The results obtained corroborates the antioxidant activity of the seed which might be due to its high amount of phenols and flavonoids and might be relevant in the prevention and management of oxidative stress-induced diseases.
Keywords: Oxidative stress; Punica granatum; Antioxidant
Oxidative stress results when there is an excess production of Reactive Oxygen Species (ROS) such as superoxide anion (O2 -), hydrogen peroxide (H2O2) and hydroxyl radical (OH-) beyond the capacity of the endogeneous antioxidant scavenging system. This leads to various complications and diseases because those reactive oxygen species exhibit deleterious effects on various molecules in the cell which eventually produces effects implicated in aging, cancer, diabetes mellitus and other cardiovascular diseases [1]. Medicinal plants, fruits, vegetables and grains are reported to be rich in various bioactive compounds such as carotenoids, vitamins, phenolic compounds and flavonoids that help to scavenge free radicals thereby protecting against oxidative stress [2]. There is the existence of a synergistic network of antioxidant enzymes such as catalase, superoxide dismutase (SOD) in vivo which acts as a defence mechanism against oxidative stress to cells. However, there are also dietary sources of antioxidants such as vitamin C [3]; vitamin A and E [4] and other endogenous molecules such as glutathione [5].
Due to the various complications of oxidative stress as a result of the deleterious effects of reactive oxygen species, there is a rising increase in the search for natural compounds with strong antioxidant properties that can help prevent and combat some of these complications.
Pomegranate (Punica granatum) belongs to the Punicaceae family is a source of nutrients and also many bioactive compounds and hence has been utilised as an antihelminthic, antimicrobial, vermifuge etc. [6,7]. Various other health benefits have been credited to pomegranates because of its potent antioxidant activities attributed to its high polyphenolic content [8]. There is an increased interest of recent in sourcing for more food and medicines from various parts of pomegranate plant. However, even though there is substantial report on the studies done on pomegranate fruit, there is a limited study on its seeds and the various bioactive components present in the seed and its in vivo antioxidant potential. There is therefore the need to investigate the potential of pomegranate seed as a natural antioxidant that can be utilised in the prevention and management of oxidative stress-induced complications and diseases. The aim of this study was to analyse the proximate, phytochemical constituents of pomegranate seeds and its antioxidant property in female albino mice.
Preparation of pomegranate seed powder
Punica granatum fruits were purchased from a fruit shop. The seeds were removed from the fruit and then oven dried at 70°C for three days. After drying, the samples were milled into powder using an electric mill.
Proximate analysis
Proximate analysis of the pomegranate seed powder was carried out to determine the crude protein, crude fibre, total ash, total carbohydrate, crude lipid and moisture content following methods described by AOAC (2000).
Preparation of P. granatum seed powder extract
200 g of the powdered Punica granatum seeds was weighed and soaked in 70% ethanol for seventy-two hours. The soaked samples were then filtered into volumetric flasks and the residue was discarded. The filtrate was placed in a flat container and was allowed to dry at ambient temperature. The resulting dry extract was stored at 4°C until when needed when the extract was mixed with 1 mL of distilled water.
Phytochemical analysis
The preliminary phytochemical analysis was conducted for the presence of bioactive compounds such as alkaloids, glycosides, tannins, saponins, flavonoids in accordance to the guidelines of Harborne [9], Hebert and Kenneth [10] and Kokate [11].
Determination of total phenolic content of the extract: Total phenolic content of the extract was done using Folin-Ciocalteu’s phenol reagent reaction as described by Singleton [12]. The calibration curve solutions were prepared by pipetting 0, 0.2, 0.4, 0.6, 0.8, and 1.0 mL of gallic acid standard solution (1.0 mg/mL garlic acid) in triplicates into clean dried test tubes. 10 mg of pomegranate seed powder extract was dissolved in 10 mL of distilled water (mg/mL) to make stock sample solution. From the stock, 0.2 mL each of 1 mg/mL was pipetted into clean dry test tubes in triplicates. The testtubes were made up to 1.0 mL with distilled water. To each of the test tube was added 1.5 mL of diluted (1:4 v/v) Folin-Ciocalteu’s reagent, incubated at room temperature for 5 minutes followed by the addition of 1.5 mL of 10% (w/v) NaHCO3 solution to give a total volume of 4.0 mL. The reaction mixtures were further incubated for an additional one and half hours and the absorbance was read at 725 nm against a blank in a spectrophotometer. The standard curve was obtained by plotting absorbance against the concentration. The concentration of the phenolic extract was determined from the standard curve and expressed in μgGAE/mL. The concentration in mg GAE/g extract was obtained using the equation below:
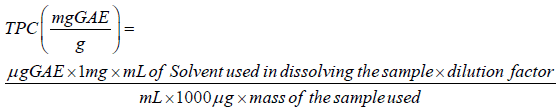
Determination of tannin content: 200 mg of pomegranate seed powder extract was weighed into a bottle and 10 ml of 70% aqueous acetone was added and properly covered. The bottle was put into an ice bath shaker and shaken for 2 hours at 30°C. Each solution was then centrifuged and the supernatant stored in ice. 0.2 ml was pipetted each into test tubes and 0.8 ml of distilled water was added. Standard tannic acid solutions were prepared from a 0.5 mg/ml stock and the solution was made up to 1 ml with distilled water. About 0.5 ml folin reagent was then added to both sample and standard followed by the addition of 2.5 ml of 29% Na2CO3. The solution was mixed and incubated for 40 minutes at room temperature after which the absorbance was read at 725 nm [13].
Determination of saponin content: The saponin content was determined in line with the method of Brunner [14]. 100 ml of isobutyl alcohol was added to 1 g of pomegranate seed powder extract in a beaker and the mixture was shaken for 2 hours to ensure uniform mixing. Thereafter the mixture was filtered into a 100 ml beaker and 20 ml of 40% saturated solution of Magnesium carbonate was added and the mixture made up to 250 ml. The mixture was again filtered to obtain a clear colourless solution. 1 ml of the colourless solution was then pipetted into a 50 ml volumetric flask and 2 ml of 5% FeCl3 solution was added and made up to mark with distilled water. It was allowed to stand for 30 minutes for blood red colour to develop. 0.1– 1.0 mg/ml standard saponin solution was prepared from saponin stock solution. The standard solution was treated similarly with 5% of FeCl3 solution as done for 1 ml of sample above. A dilution of 1 to 10 was made from the prepared solution. The absorbance of the samples as well as that of the standard solution was read after colour development in a spectrophotometer at a wavelength of 380 nm.
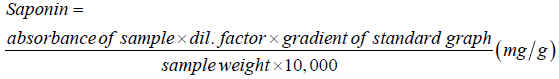
Determination of total flavonoids content: The flavonoid content in the pomegranate seed powder extract was determined spectrophotometrically according to the procedure of Bohm and Koupai Abyazani [15]. 0.01 g of the extract was dissolved in 5mL of methanol and made up to 20 mL to give a final concentration of 0.5 mg/ml. To clean, dry test tubes (in triplicate) were pipetted 0.2mL of working solution of the sample and diluted with 4.8 mL distilled water. To each test tube was then added 0.3 mL of 5% (w/v) NaNO2, 0.3 mL of 10% AlCl3 and 4 mL of 4% (w/v) NaOH. The reaction mixtures were incubated at room temperature for 15 minutes. The absorbance was read at 500 nm against reagent blank containing all reagents except the extract or standard catechin in the case of standard curve solutions. The standard calibration curve was prepared by pipetting 0.0, 0.2, 0.4, 0.6, 0.8, 1.0 ml of 1 mg/mL catechin into clean dry test tubes. The volumes were made up to 5 mL with distilled water. To each of the tubes were added 0.3 mL of 5% (w/v) NaNO2, 0.3 mL of 5% (w/v) AlCl3 and 4 mL of 4% (w/v) NaOH. The reaction mixture was incubated at room temperature for 15 minutes. Absorbance was taken at 500 nm and was plotted against the concentration to give the standard calibration curve. The concentrations of the flavonoids in the extract was extrapolated from standard calibration curve and expressed as milligram catechin (CAT) equivalent per g of extract (mg CAT/g extract).
The value extrapolated from the standard curve gave the concentration in μg CAT/mL. The concentration in mgGAE/g extract was obtained using the equation below:
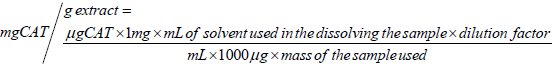
Experimental animals
A total of sixteen adult female wistar albino rats were allowed to acclimatize under the same condition for two weeks. The animals were kept in clean cages and maintained under the standard laboratory condition for temperature (26 ± 6°C, humidity (60 ± 5%) and controlled environment (5:12 h light/dark cycle). They were given free access to standard pellet and water. All the experimental procedures were carried out in accordance to the guidelines of the Institutional Animal Ethics Committee (IAEC).
Experimental design
At the end of the acclimatization period, the sixteen rats were randomly divided into four groups, with four rats in each. The rats in:-
Group I- was the control group fed on pellet and water only;
Group II- received 200 mg/kg per body weight of P. granatum seed extract orally for 14 days.
Group III- received 300 mg/kg per body weight of P. granatum seed extract orally for 14 days.
Group IV- received 400 mg/kg per body weight of P. granatum seed extract orally for 14 days.
Measurement of body weights
The initial and final body weights of the animals were taken before and after the experiment using a weighing balance. The weight gain was then extrapolated from the initial and final weight values.
Assessment of the in vivo antioxidant activity of P. granutum seed extract
At the end of the experiment, the animals were placed on overnight fasting for 12 hours and then sacrificed under light ether anaesthesia by cervical dislocation. Blood samples were collected by direct cardiac puncture. The serum was separated from the blood by centrifuging at 2500 rpm for 15 minutes and the levels of the following antioxidant parameters were determined from the serum:
Determination of catalase (CAT): Catalase activity was determined in the serum using Aebi’s method [16]. 50 microliter of the sample was added to a cuvette containing 450 μL of phosphate buffer (0.1M, pH 7.4) and 500 μL of 20 mM H2O2. Catalase activity was measured at 240 nm for 1 minute using spectrophotometer. The molar extinction coefficient of H2O2, 43.6 M cm−1 was used to determine the catalase activity. One unit of activity is equal to 1 mmol of H2O2 degraded per minute and was expressed as units per milligram of protein.
Calculation:
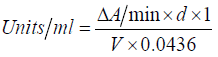
d = dilution of original sample for Catalase Reaction
V = Sample volume in Catalase Reaction (ml)
0.0436 = εmM for hydrogen peroxide
1 = Total reaction volume
Determination of Superoxide dismutase: The method used was described by Mccord and Fridovich [17]. To 200 μL of the lysate, 2.5 ml of 75 mM of Tris–HCl buffer (pH 8.2), 30 mM EDTA and 300 μL of 2 mM of pyrogallol was added. An increase in absorbance was recorded at 420 nm for 3 minutes by spectrophotometer. One unit of enzyme activity is 50% inhibition of the rate of auto oxidation of pyrogallol as determined by change in absorbance/min at 420 nm. The activity of SOD was expressed as units/mg protein.
Calculation:
Increase in absorbance per minute = A3 – A0 / 2.5
Where A0 = absorbance after 30 seconds
A3 = absorbance after 150 seconds
% inhibition = 100 – 100 X (increase in absorbance for substrate/increase in absorbance for blank)
1 unit of SOD activity was given as the amount of SOD necessary to cause 50% inhibition of the oxidation of adrenaline.
Determination of glutathione (GSH): The reduced glutathione (GSH) content of blood tissue as non-protein sulphydryl was determined according to the method described by Sedlak and Lindsay [18]. To the serum, 10% TCA (Trichloro Acetic Acid) was added and centrifuged. 1.0 ml of supernatant was treated with 0.5 ml of Ellman’s reagent (19.8 mg of 5, 5-dithiobisnitro benzoic acid (DTNB) in 100 ml of 0.1% sodium nitrate) and 3.0 ml of phosphate buffer (0.2M, pH 8.0). The absorbance was read at 412 nm.
Statistical analysis: All data were subjected to statistical analysis of variance, and means was separated according to their significant differences using the Duncan multiple Range Test. A value of p<0.05 was considered statistically significant when compared to the control group.
Proximate composition of pomegranate seed (Punica granatum)
The result in Table 1 shows the results obtained from the proximate composition analysis of P. granatum. As shown in the table, the highest value was obtained for fiber, followed by fat, carbohydrate, protein and moisture and ash.
| Parameter (%) | (Punica granatum) seed |
|---|---|
| Moisture | 6.84 ± 0.03 |
| Ash | 1.55 ± 0.04 |
| Fibre | 27.59 ± 0.27 |
| Protein | 14.06 ± 0.18 |
| Fat | 26.03 ± 0.09 |
| Carbohydrate | 23.96 ± 0.14 |
Table 1: Proximate composition of pomegranate seed powder.
Body weight
Table 2 shows the result obtained from the measurement of the initial and final body weights of the mice. There was an increase in the body weight of all the animals that received P. granatum extract. However, it was the group that received 200 mg/kg P. granatum extract that had the highest percentage weight gain (31.5%).
| Group | Mean Initial body weight before treatment (g) | Mean final body weight after 14 days of treatment with P. granatum extract (g) | Weight gain (g) |
|---|---|---|---|
| Control | 114.97 ± 0.05 | 137.55 ± 0.02 | - |
| Group I (200 mg/kg) P. granatum extract |
92.48 ± 0.01 | 121.62 ± 0.03 | 29.14 |
| Group II (300 mg/kg) P. granatum extract |
94.38 ± 0.02 | 116.58 ± 0.03 | 22.20 |
| Group III (400 mg/kg) P. granatum extract | 114.99 ± 0.01 | 121.70 ± 0.02 | 6.71 |
Table 2: Body weight.
Phytochemical analysis
The result obtained from the phytochemical analysis of P. granatum seed extract reveals the presence of flavonoids which was present in the highest amount (121.22 mg/g); followed by phenol (78.12 mg/g), saponins (12.87%), tannins (3.30 mg/g) and alkaloid (1.51 mg/g) (Table 3).
| Phytochemical (mg/g) | P. granatum seed extract |
|---|---|
| Phenol | 78.12 ± 0.91 |
| Tannin | 3.30 ± 0.10 |
| Saponin | 12.87 ± 0.01 |
| Flavonoids | 121.22 ± 0.65 |
| Alkaloids | 1.51 ± 0.05 |
Table 3: Quantitative phytochemical components of Pomegranate seed extract.
Effect of P. granatum seed extract on the serum levels of GSH, SOD and CAT in experimental animals
As shown in Table 4, there was a significant (p<0.05) increase in the levels of GSH and catalase of all the groups that received the extract when compared to the control group while the highest increase was observed in Group III (400 mg/kg). There was no significant (p>0.05) increase in the serum SOD level of the rats in Group I (200 mg/kg) and Group II (300 mg/kg) as compared to the control. However, there was a significant (p<0.05) increase in the SOD level of rats in group III (400 mg/kg) when compared to that of the control group.
| Antioxidant parameter (µmol/ml/min) |
Control group | Group I (200 mg/kg) |
Group II (300 mg/kg) |
Group III (400 mg/kg) |
|---|---|---|---|---|
| Glutathione (GSH) | 45.73 ± 2.01 | 50.77 ± 1.58⃰ ⃰ | 54.04 ± 1.35⃰ ⃰ ⃰ ⃰⃰ | 59.20 ± 1.32⃰ ⃰ ⃰ ⃰ |
| Superoxide dismutase (SOD) | 304.9 ± 11.31 | 329.1 ± 73.93 | 401.2 ± 50.75 | 409.7 ± 61.09⃰ |
| Catalase (CAT) | 578.3 ± 22.93 | 713.3 ± 4.60⃰ ⃰ ⃰ ⃰ | 710.7 ± 30.17⃰ ⃰ ⃰ ⃰ | 717.7 ± 21.41⃰ ⃰ ⃰ ⃰ |
Table 4: Effect of P. granatum seed extract on the serum levels of antioxidant parameters (GSH, SOD and CAT) in mice.
In this study, the proximate, phytochemical analysis and the antioxidant effect of pomegranate seed in mice was evaluated. Proximate analysis of the seed shows that the seed contains 27. 59% crude fiber, 23.96% carbohydrate, 26.03% crude fat, 14.06% protein, 6.84% moisture and 1.55% ash. Pomegranate seeds are considered a good source of crude fibre, crude fat, carbohydrates and protein and therefore can be utilized as food supplements. P. granatum seeds when compared to date palm seed however a lower fiber has content [19]. Crude fiber provides numerous health benefits to the body as it has ability to decrease serumlow density lipoprotein (LDL) cholesterol level [20].
As can be seen also from the result, pomegranate seeds contain considerable amounts of protein, crude fat and carbohydrate. Crude protein serves as a source of fuel and it is a nutrient needed by the human body for growth and maintenance [21]. Crude fats provide important energy storage in the body and therefore an especially important food component when higher storage is required and also serve as significant food supplement [22]. Carbohydrates provide energy, as they are the body’s main source of fuel, needed for physical activity, brain function and operation of the organs. They are also important for intestinal health and waste elimination [23]. Crude ash is an index for the mineral content of a food sample as minerals are required for metabolic processes and for oxygen transport in the body system [24].
The phytochemical analyses of the extract of the pomegranate seed indicated the presence of flavonoids, tannins, saponins, alkaloids, and phenols. Phenol and phenolic-like compounds such as flavonoids are known to possess significant antioxidant activities [25]. The pomegranate seed has high phenolic content (flavonoids). In a previous report on the chemical composition of pomegranate seeds by Salim [26], they reported a higher content of phenol and a lower content of flavonoids in the fresh pomegranate seed. This discrepancy might be due to various pomegranate cultivars and maturity which led to the variation in the biosynthesis of phenolic metabolites [27]. Another justification is due to the different solvents used for extraction [28]. According to a previous study, methanol extract yields the maximum antioxidant activity among methanol, acetone or water when used for the extraction of antioxidants from pomegranate [29].
Previous studies have shown that flavonoids and phenolics prevent oxidative cell damage, possess potent anticancer activities due to the fact that they might induce mechanism that affect cancer cells and inhibit tumour invasion [30] by scavenging reactive oxygen species [31,32]. This can also be attributed to the fact that they possess conjugated ring structures and carboxylic groups which helps to inhibit lipid peroxidation. The anti-oxidative properties of the plant polyphenols are thought to arise from their reactivity as hydrogen or electron donors from their ability to stabilize unpaired electrons and to terminate fenton reactions [33].
Pomegranate seed also contains saponins, tannins and alkaloid. The presence of saponins in medicinal plants have been attributed to most biological effects related to cell growth and division in humans and have inhibitory effect on inflammation [34]. Studies have shown that saponins have anti-tumor and anti-mutagenic actions and can reduce the risk of human cancers, by thwarting the growth of cancer cells [35]. The non-sugar part of saponins also possesses a direct antioxidant activity, which may result in other benefits such as reduced risk of cancer and heart diseases [5]. Tannins are phenolic compounds of high molecular weight that are caustic in nature and promote wound healing. Pomegranate seeds have been reported to possess excellent healing properties, ability to protect the outer layers of the skin and aid in the regeneration of the cells [36]. Plants rich in alkaloids are known to possess antimicrobial activity [37].
Results obtained from this study also show significant effects on both the non-enzymatic and enzymatic antioxidant indices. Glutathione conjugation serves as protective mechanism whereby potentially toxic metabolites are “mopped up” as cellular redox status [38]. There was a significant (p<0.05) increase in the level of GSH in the mice that received P. granatum extract when compared to the control group. The antioxidant enzymes; catalase and suproxide dismutase (SOD) represent the primary intracellular antioxidant defense mechanism against oxidative stress [39]. There was a significant (p<0.05) increase in the SOD level of the mice in the group that received 400 mg/kg group P. granatum extract when compared to the control group.
Catalase, a tetrametric hemoprotein is present in the liver cells and erythrocytes at high concentration [40]. It scavenges the hydrogen peroxide H202 generated from the liver cell generated by the action of SOD. There was a significant (p<0.05) increase in the catalase levels in all the groups that received P. granatum extract when compared with the control group.
It has been reported that pomegranate extracts scavenge free radicals and decrease macrophage oxidative stress and lipid peroxidation in animals [41] and increase plasma antioxidant capacity in elderly humans [42]. A study conducted on rats given pomegranate by-product (PBP) extract made from whole fruit showed a 53-percent increase in their glutathione levels which further confirm the antioxidant properties of pomegranate [41]. Also, a separate study in rats with CCl4- induced liver damage demonstrated that pre-treatment of those rats with pomegranate peel extract (PPE) enhanced the freeradical scavenging activity of the hepatic enzymes catalase, superoxide dismutase, and peroxidase, and resulted in 54-percent reduction of lipid peroxidation values compared to controls [43].
The antioxidant property demonstrated by pomegranate seed extract might also be attributed to the various nutritive contents most especially its high fiber content as fiber has been linked to improved levels of SOD and CAT activities in rats as reported by Tingting [44] and also the high amounts of phenols and flavonoids present in the seed extract. Phenolics are known as potential chemopreventive agents and are essential for counteracting oxidation stress; possess potent free radicals scavenging and antioxidant properties [45]. Antioxidant activity may be linked to diverse phenolic compounds present in pomegranate including punicalagin isomers, ellagic acid derivatives and anthocyanins (delphinidin, cyanidin and pelargonidin 3-glucosides and 3, 5 diglucosides). These compounds are known for their properties in scavenging free radicals and inhibiting lipid oxidation [8,46].
This study confirms the antioxidant activity of Punica granatum seeds which might be attributed to its phytochemical components most especially flavonoids and phenols. This property might make it a potential agent that might be used to prevent and manage the complications of oxidative-stress induced diseases. However, further studies should be carried out to isolate the specific bioactive compounds in pomegranate seed and to elucidate fully the mechanism of its in vivo antioxidant effect.