Journal of Cell Science & Therapy
Open Access
ISSN: 2157-7013
ISSN: 2157-7013
Research Article - (2023)Volume 14, Issue 4
Objective: To verify proteomic evidence for fibrinolysis and Neovascularization (NV) in patients with Chronic Limb Threatening Ischemia (CLTI) treated with a novel cell therapy approach.
Methods: Six CLTI patients were treated for one month with filgrastim (Amgen Inc) 10 mcg/kg SQ every 72 hours, and an external Programmed Infra-Geniculate Compression Pump (PCP) for 3 hours a day. Blood was drawn on day 1 (baseline), and on days 15 and 30 (24 hours after the 5th and 10th Filgrastim doses). On each of these days blood was sampled before and after 2 hours of supervised PCP. Serum ELISA was used to measure the concentration of Plasmin, Fibrin Degradation Products (FDP), VEGF-A, Hepatocyte Growth Factor (HGF) and MMP-9.
Results: Statistically significant elevation of plasmin and FDP (p<0.001) were consistently measured 24 hours after each Filgrastim administration and was not influenced by the pump. No hemorrhagic complications occurred. Pump independent significant elevation in VEGF-A, HGF and MMP-9, each associated with NV, was also observed.
Conclusion: These observations support angiographic, hemodynamic and clinical evidence of fibrinolysis and neovascularization achieved with this novel therapy. These data support further clinical testing of this cell therapy in CLTI, as well as in other ischemic tissue beds.
G-CSF; Chronic limb threatening ischemia; Fibrinolysis; Neovascularization; Fibrin degradation products; Plasmin; Hepatocyte growth factor; Art assist; Cell therapy; Amputation; Ischemia; Filgrastim; Neupogen
In the presence of arterial occlusive disease, Neovascularization (NV) is a natural process that strives to preserve tissue perfusion through growth of collateral arteries (arteriogenesis) and growth of capillaries, arterioles and venules (angiogenesis). Endothelial shear stress initiates arteriogenesis, while hypoxia stimulates angiogenesis. NV becomes impaired as vascular disease progresses, leading to Chronic Limb Threatening Ischemia (CLTI). Symptoms include forefoot ischemic rest pain, ulceration, and/or gangrene. Invasive revascularization (surgery or catheter based) is typically offered to prevent amputation.
Despite significant procedural risk, variable durability and high cost, these approaches remain the standard of care [1].
Our premise is that NV is the safest and most durable revascularization. Our approach differs from Phase II clinical trials of cell therapies for CLTI recently performed. We simply improve the ischemic environ so NV is restored, rather than trying to micro-manage the process. This eliminates the need for injection of specific agents or cells into the ischemic tissues, exvivo cell processing, and concerns about cell distribution and survival following injection. To do this, we address 7 obstacles to a permissive NV environment in CLTI. The first 5 obstacles are traffic based impairments. Multilevel arterial occlusions 1) attenuate the endothelial shear stress stimulus needed to initiate arteriogenesis, 2) impair delivery of oxygenated nutritive blood flow, 3) inhibit efficient clearance of toxic metabolic byproducts, 4) hinder dissemination of protein distress signals (e.g. SDF-1) and 5) impede the arrival of monocytes and mobilized salutary progenitor cells to the distressed tissue. The result is a sub-optimal microenvironment for biochemical processes. Together, these 5 obstacles are addressed with a Programmed Compression Pump (PCP) below the knee. We used the ArtAssist device (ACI Medical, San Marcos, CA) in CLTI patients for a minimum of 3 hours daily in the seated position [2].
When this project was initiated by D Eton in 2008, literature suggested the severity of arterial disease correlated with a sixth obstacle: The diminished number and function of circulating progenitor cells. Filgrastim was selected to overcome this obstacle. Filgrastim is a Granulocyte Colony Stimulating Factor (G-CSF) that is FDA approved for stem cell mobilization from bone marrow niches. A novel dosimeter was used: Filgrastim was injected subcutaneously at a dose of approximately 10 mcg/kg once every 72 hours, for a total of 10 doses over one month.
The debate about paracrine vs direct progenitor cell incorporation in vascular remodeling and growth was already being waged when D Eton obtained data in the first 2 patients treated with the PCP and filgrastim at the university of Miami. Corkscrew collaterals were seen on arteriography, and hemodynamics improved (increase in ankle brachial index, development of pulsatility in the forefoot and toes). There was abatement of ischemic rest pain, and healing of forefoot ischemic wounds. Most importantly, thrombus was being cleared from segmentally occluded arteries, as well as the microcirculation. Arteriography demonstrated segmental recanalization of previously occluded infrageniculate arteries and significant improved contrast transit (Figure 1). Overcoming the seventh obstacle, chronic occlusive thrombus in small and large arteries, with G-CSF was a new discovery. Filgrastim at a novel dosimetry induced physiologic fibrinolysis over 30 days without a serious hemorrhagic complication, in addition to inducing a pro-angiogenic milieu. Independent proteomic confirmation is the purpose of this study [3].
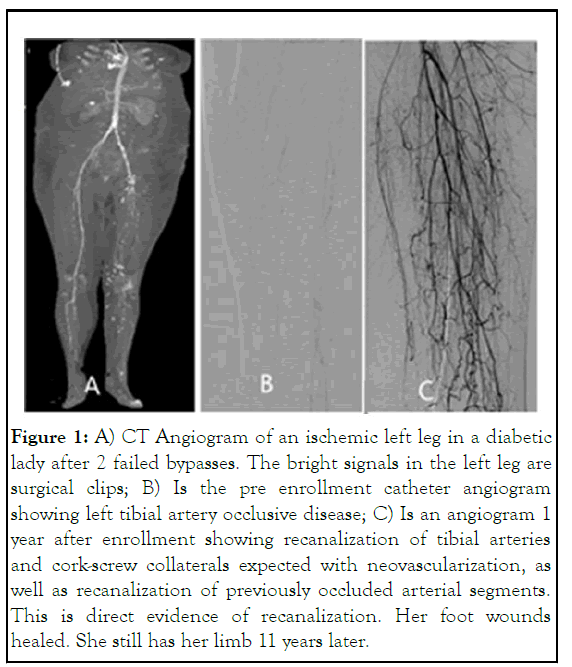
Figure 1: A) CT Angiogram of an ischemic left leg in a diabetic lady after 2 failed bypasses. The bright signals in the left leg are surgical clips; B) Is the pre enrollment catheter angiogram showing left tibial artery occlusive disease; C) Is an angiogram 1 year after enrollment showing recanalization of tibial arteries and cork-screw collaterals expected with neovascularization, as well as recanalization of previously occluded arterial segments. This is direct evidence of recanalization. Her foot wounds healed. She still has her limb 11 years later.
Six CLTI patients aged 57 to 84 were enrolled in a single arm IRB approved trial at university of Illinois Chicago in 2017-18. Inclusion criteria included patients complaining of >2 weeks of ischemic forefoot rest pain, gangrene and/or ischemic ulceration, with confirmatory hemodynamic testing (ABI<0.45). Exclusion criteria are listed in Table 1. While the FDA label for filgrastim does list progenitor cell mobilization as an indication, use of filgrastim to improve NV and to induce fibrinolysis are not on the filgrastim label. The FDA granted a clinical research waiver.
| Exclusion label of filgrastim | ||
| Acute limb ischemia requiring emergency treatment | Body mass index>34 | Uncorrected symptomatic coronary artery disease |
| Non-salvageable foot (e.g. extensive gangrene, advanced infection, rigor mortis, knee/hip flexion contracture post-stroke paralysis, and hemiparesis) | Severe venous insufficiency causing venous stasis ulceration and dermatitis | History of lymphoma or leukemia |
| Untreated hypercoagulability disorder, sickle cell anemia, myeloproliferative disorder | Uncorrected significant aorto-iliac, common femoral and profunda femoral arterial disease | - |
| Dialysis, or sustained elevated creatinine>4 mg/dl | Ulceration precluding PPCD placement | - |
| Severe dementia, bed-ridden, non-compliance, unlikely to follow-up, unreliable | Active cancer | - |
| Intolerance of PPCD compression | Allergy to neupogen | - |
Table 1: Exclusion criteria.
Following informed IRB approved consent, patients were brought to the UIC Clinical Research Center (CRC) where baseline phlebotomy was performed. Each patient was instructed on use of the Programmed Pneumatic Compression device (PCP: ArtAssist, ACI medical San Marcos, CA). Each was then observed during 2 hours of continuous PCP use, whereupon another blood sample was obtained. Refrigerated filgrastim (approximately 10 mcg/kg) was then administered subcutaneously in the lower abdominal wall. Each patient was observed for adverse effects before being instructed to return home to use PCP daily for 3 hours in the seated position during the month of the study. Approximately every 72 hours the medication was re-administered over 30 days. Each patient served as his/her own control [4]. Filgrastim (Neupogen) was obtained refrigerated from amgen Inc (Thousand Oaks, CA). It was stored until use in the carton to protect from light at between 2°C to 8°C (36°F to 46°F).
Filgrastim was packaged in vials of either 480 mcg or 300 mcg. The cost of the product and maintenance of sterility precluded partial use of a third vial. Additionally there were small losses of agent during administration, which typically required two separate injections in the subcutaneous tissue in the lower abdomen. True dose estimates range from 8 mcg/kg to 10 mcg/kg. Filgrastim is a potent agent with a half-life between 3 and 4 hours. The peak Increase in circulating leukocytes was observed at approximately 24 hours and returned to normal by 72 hours.
The art assist device provides programmed sequential rapid inflation (0 to 120 mmHg in <0.3 sec) of leg cuffs that apply pressure to the calf, ankle, and foot. This provides the shear stress stimulus, while at the same time driving in oxygenated nutritive blood flow and facilitating venous return. The pressure is held in each of the cuffs for three seconds. Rapid deflation follows, thus applying a second endothelial shear stress stimulus. Three cycles occur per minute. PCP was continued at home on both legs in the seated position for 3 hours a day. While this study was for 30 days, patients had the option to continue PCP until improvement of symptoms reached a plateau (typically less than 5 more months).
Phlebotomy two pairs of blood specimens were obtained from an antecubital vein with a 21-gauge butterfly at the CRC at 3 time points in serum separator tubes. A pair includes phlebotomy prior to and immediately after two hours of supervised PCP use at the CRC. As mentioned above, the first pair was obtained on enrollment in the study. The second pair was drawn one day after the 5th G-CSF dose. The third pair was drawn one day after the 10th (and final) G-CSF dose.
Enzyme-Linked Immunosorbent Assay (ELISA)
The blood samples were collected in a serum separator tube. Following clot formation (30 min) each tube was centrifuged for 15 min at 1000 g and the separated serum was stored at -20°C until batch analysis. ELISA was performed according to the manufacturer’s instructions in the research laboratory of author amelia Bartholomew. To reduce variance serum was analyzed with a single ELISA kit for each protein. Fibrinolysis was assessed by measuring plasmin and Fibrin Degradation Products (FDP). Stimulation of endothelial growth was assessed by measuring Vascular Endothelial Growth Factor A (VEGFA) and Hepatocyte Growth Factor (HGF). Proteolytic activity was assessed by measuring Membrane Metalloproteinase-9 (MMP-9), which has two relevant roles: Subendothelial matrix reorganization as well as helping to free up the mobilized progenitor cells in the bone marrow [5].
Statistics
The impact of filgrastim 24 hours after the 5th and 10th Filgrastim doses on proteins involved in fibrinolysis and in NV is reported. Blood is drawn before and after 2 hours of PCP to assess PCP impact. Each patient is his/her own control. The mean, standard deviation and p values obtained by the paired ttest are tabulated (Table 2). The standard deviations reflect the diversity of patients with co-morbidities of advanced atherosclerosis (hypertension, hyperlipidemia, history of tobacco use, diabetes, genetic pre-disposition). Despite this, and the presence of other co-morbidities (e.g. COPD, mild azotemia), the effect of filgrastim on the proteins measured was readily discernible.
| 2019 UIC DATASET 6 patients | ||||||||||
| Concentration ELISA (pg/ml) (Mean(SD)) | ||||||||||
| Day 1 (Mean (SD)) | Day 14 (Mean (SD)) | Day 30 (Mean (SD)) | % Increase vs day 1 (%(SD)) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| t=0 | t=2 hours | t=0 | t=2 hours | t=0 | t=2 hours | Day 14 | P-value | Day 30 | P-value | |
| Plasmin | 177 (244) | 103 (45) | 1158 (494) | 939 (239) | 1311 (260) | 1202 (429) | 1225 (1055) | 0.001 | 1384 (965) | <0.001 |
| FDP | 7.8 (6.6) | 5.1 (3.1) | 53 (23) | 42 (11) | 51 (18) | 46 (19) | 762 (503) | 0.003 | 733 (353) | 0.002 |
| HGF | 994 (211) | 955 (229) | 4258 (1633) | 3616 (1259) | 4391 (1701) | 4218 (502) | 316 (169) | 0.003 | 413 (152) | 0.003 |
| VEGFA | 192 (194) | 199 (193) | 305 (224) | 295 (224) | 403 (216) | 384 (190) | 73 (45) | 0.008 | 169 (138) | 0.005 |
| MMP-9 | 308 (230) | 295 (218) | 1680 (488) | 1509 (489) | 1940 (285) | 1956 (353) | 759 (733) | 0.001 | 979 (909) | 0.005 |
Table 2: ELISA results for Combination therapy (Filgrastim 10 mcg/kg SQ every 72 hours+PCP) in 6 patients treated at university of Illinois at Chicago between 2016 and 2018. There is significant increase in expression of 2 markers of fibrinolysis (plasmin, FDP), as well as a significant increase in 2 proteins associated with endothelial growth (HGF, VEGFA) and a significant increase in MMP-9, associated with sub-endothelial matrix proteolysis.
The original highly significant (p<0.01) proteomic and cytometry data obtained between 2013-2015 (DE, GZ, TCH) supported the sample size of 6 patients to look at plasmin, FDP, VEGFA, HGF, and MMP-9, providing 80% power to detect changes in protein levels of 0.66 standard deviations assuming a two-sided significance level of 0.05. These hereto unpublished historical data are provided for review in the appendix. Additionally proteomic and cytometry data (hereto unpublished) from a historical control group of 19 CLTI patients treated with PCP alone (no drug) over a 30 day period in a 2012-2014 university of Chicago IRB approved trial is included in the appendix [6].
Fibrinolysis
A statistically significant twelve-fold elevation of serum plasmin was measured 1 day after the 5th G-CSF injection, and nearly 14-fold increase 1 day after the 10th dose (Table 2), both referenced to the serum plasmin measured on day 1 (baseline). Moreover, an approximately 7.5 fold increase in serum FDP was measured. These data are consistent with the recanalization observed of chronically occluded arterial segments and the improved contrast transit time on follow-up angiograms observed in CLTI patients treated with this novel combination therapy. These independently obtained data (different institution, investigators, equipment) confirm the original 2013-2015 findings from university of Chicago.
Neovascularization
VEGFA, HGF and MMP-9 increased significantly 1 day after both the 5th and 10th G-CSF injections in Table 3. The first two promote endothelial growth during NV. MMP-9 is one of the proteolytic proteins associated with the matrix reorganization during NV, to make room for the widening and elongating vessels (“cork-screw collaterals” observed angiographically). MMP-9 is also associated with assisting in progenitor cell mobilization from the marrow into the blood stream. These independently obtained data confirm the original 2013-2015 findings from university of Chicago (DE, GZ, TCH). Platelet Derived Growth Factors (PDGF-AA and PDGF-BB) involved in vascular smooth muscle cell growth within the media layer of the arterial wall were significantly elevated 1 day after the 5th and 10th G-CSF injections. Transforming Growth Factor beta (TGFb) and Tumor Necrosis Factor (TNF) also increased significantly. These findings were pump independent.
Angiopoietin 1 did not increase significantly in the data from the UIC experiment. This differs from the 2015 study where a significant (P<0.05) increase was observed 1 day after the 5th GCSF dose (Table 3), but not after the 10th dose. With such large standard deviations, the sample size is too small to validate these findings in either experiment, as well as the other measurements in Table 3 where non-significant increases (pump independent) were measured (Placental growth factor, PDGF-AB, Interleukin-6 (IL6), Insulin Growth Factor-1 (IGF-1) [7].
| 2015 UC DATASET8 patients | ||||||||||
| Concentration ELISA (pg/ml) (Mean (SD)) | ||||||||||
| Day 1 (Mean (SD)) | Day 14 (Mean (SD)) | Day 30 (Mean (SD)) | % Increase vs Day 1 (%(SD)) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| t=0 | t=2 hours | t=0 | t=2 hours | t=0 | t=2 hours | Day 14 | P-value | Day 30 | P-value | |
| Plasmin | 298 (231) | 382 (246) | 662 (39) | 543 (65) | 645 (31) | 644 (93) | 1071 (1999) | 0.003 | 1394 (2308) | 0.03 |
| FDP | 3.4 (3.3) | 3.7 (3.3) | 7.6 (3) | 5.5 (2.6) | 7.8 (1.3) | 7.5 (2.7) | 439 (646) | <0.001 | 421 (642) | 0.01 |
| HGF | 2403 (1256) | 3532 (2983) | 7528 (3750) | 8122 (3759) | 7428 (3375) | 8178 (3523) | 234 (244) | 0.001 | 430 (396) | 0.01 |
| VEGFA | 345 (252) | 399 (264) | 587 (181) | 597 (181) | 758 (404) | 755 (360) | 735 (1568) | 0.05 | 1218 (1960) | 0.05 |
| MMP-9 | 820 (619) | 1305 (947) | 2215 (329) | 2144 (449) | 2257 (517) | 2152 (580) | 410 (637) | 0.001 | 549 (644) | 0.02 |
| PDGF AA | 2753 (2653) | 2634 (2505) | 4356 (1833) | 4623 (1853) | 4520 (527) | 4650 (2054) | 740 (1477) | 0.02 | 1065 (1656) | 0.05 |
| PDGFBB | 1699 (989) | 1683 (835) | 3110 (2130) | 2661 (792) | 3323 (985) | 3435 (1075) | 225 (284) | 0.04 | 224 (257) | 0.02 |
| TNF | 4 (2) | 4.4 (1.5) | 2.18 (1.9) | 5.9 (2) | 6.4 (1.9) | 4.6 (1.8) | 55 (80) | 0.05 | 80 (99) | 0.04 |
| TGFb | 1009 (320) | 1123 (343) | 1458 (572) | 1575 (543) | 1550 (247) | 1455 (323) | 56 (67) | 0.04 | 58 (76) | 0.05 |
| Angiopoietin | 19465 (18115) | 19833 (19461) | 40026 (20484) | 41588 (19530) | 51288 (12756) | 42322 (25399) | 1345 (2196) | 0.05 | 1585 (2531) | 0.1 |
| IGF-1 | 82 (41) | 91 (45) | 106 (43) | 105 (42) | 108 (44) | 111 (34) | 48 (63) | 0.08 | 47 (36) | 0.08 |
| PDGF AB | 145 (99) | 139 (96) | 217 (102) | 237 (89) | 315 (99) | 309 (105) | 228 (348) | 0.1 | 338 (451 | 0.25 |
| IL 6 | 44 (88) | 40 (73) | 35 (59) | 33 (52) | 65 (118) | 68 (111) | -28 (34) | 0.2 | 71 (163) | 0.9 |
| PLGF | 32 (11) | 32 (7) | 42 (26) | 36 (15) | 38 (18) | 40 (17) | 21 (45) | 0.3 | 54 (38) | 0.1 |
| MCP-1 | 258 (121) | 352 (160) | 242 (103) | 274 (104) | 194 (105) | 241 (116) | -17 (29)0.04 | 0.04 | -28 (23) | 0.04 |
Table 3: ELISA results for combination therapy (Filgrastim 10 mcg/kg SQ every 72 hours+PCP) in 8 patients treated at university of Illinois at Chicago between 2013 and 2015. Note the elevation in all the markers for neovascularization, and the increase in FDP and plasmin, the 2 markers for fibrinolysis. Note the drop in MCP-1 with Filgrastim, which returned back to baseline after 2 hours of PCP.
Influence of PCP on NV and fibrinolysis
The fibrinolytic and NV proteomic analysis data are pump independent. There was no statistical difference between plasmin, FDP, HGF, VEGF-A, MMP-9 serum protein concentrations before and after 2 hours of pump use on Days 1, 15 and 30. The absence of a pump contribution to fibrinolysis and NV was also observed in the original study, as well as in the 19 patients treated with pump alone.
Endothelial cell activation
A premise for PCP is that without it there is insufficient shear stress caudal to multi-level arterial occlusive disease for arteriogenesis to be even initiated. Increased shear stress is reported to “activate” the endothelium, resulting in upregulation of nitric oxide synthase, expression of surface adhesion molecules, and secretion of Monocyte Chemoattractant Protein-1 (MCP-1). An activated endothelium initiates arteriogenesis.
PCP increased the serum concentration of markers of endothelial activation. There was a 12+14% and a 17+17% increase in CD31 positive cells (adhesion molecule PECAM-1) measured by cytometry (counts per 10,000 cells) on day 14, and on day 30 respectively. These data are relative to blood drawn prior to starting the PCP on day 1, and are the cumulative result of 3 hours of daily home use of the PCP for a month. Serum MCP-1 was measured by ELISA. Two hours of PCP alone (no drug) resulted in a statistically significant 16% increase in MCP-1 expression. Serum nitrite consistently increased after 2 hours of PCP. Serum nitrite is a breakdown product of nitric oxide produced by nitric oxide synthase [8].
Importantly, filgrastim caused a 10 to 30 percent (P=0.04) drop in the serum concentration of MCP-1 24 hours after each GCSF injection prior to 2 hours of supervised PCP use. This finding was pump dependent, associated with a 10-30% increase (p<0.05) in MCP-1 after 2 hours of pump use (p<0.05). 5- Cytometry (counts per 10,000), and cell blood count after GCSF (data from 2012-14: DE, GZ, TCH) (Table 4).
| t=0 | t=2 hours | % Increase | P-value | |||
|---|---|---|---|---|---|---|
| N | pg/ml (SD) | N | pg/ml (SD) | |||
| MCP-1 | 27 | 288 (111) | 27 | 335 (128) | 0.16 | 0.006 |
| FDP | 8 | 3.4 (3.2) | 8 | 4.1(3.2) | 0.22 | 0.54 |
| Plasmin | 18 | 176 (206) | 18 | 213 (247) | 0.21 | 0.26 |
| HGF | 29 | 2245 (1835) | 29 | 2583 (2367) | 0.15 | 0.18 |
| TGF-b | 29 | 1959 (1119) | 29 | 1884 (1205) | -0.04 | 0.66 |
| IGF-1 | 19 | 67 (33) | 19 | 71 (37) | 0.06 | 0.19 |
| VEGFA | 28 | 144 (199) | 28 | 162 (223) | 0.13 | 0.2 |
| PLGF | 29 | 21.5 (10.2) | 29 | 21.8 (9.9) | 0.01 | 0.77 |
| TNF | 29 | 3 (2) | 29 | 3.2 (2) | 0.08 | 0.09 |
| IL-6 | 28 | 14(16) | 28 | 17 (21) | 0.21 | 0.008 |
| Angiopoitein-1 | 18 | 11179 (15096) | 18 | 11309 (16006) | 0.01 | 0.84 |
| PDGF-AA | 29 | 1361 (1831) | 29 | 1257 (1793) | -0.08 | 0.16 |
| PDGF-BB | 29 | 1103 (765) | 29 | 1125 (771) | 0.02 | 0.83 |
| PDGF-AB | 18 | 88 (83) | 19 | 89 (79) | 0.02 | 0.74 |
| MMP-9 | 29 | 447 (474) | 29 | 591 (732) | 0.32 | 0.14 |
Table 4: PCP Alone before and after 2 hours in 19 patients (2012-2014).
The number of circulating CD34+ progenitor cells and VEGFR2+ endothelial progenitor cells increased 44%+42% (p<0.001) and 68%+21% (p<0.001), respectively, 1 day after the last (10th) G-CSF injection as compared to day 1. The number of leukocytes, neutrophils, and monocytes increased a range of 3 to 7 fold, 2 to 7 fold, and 1 to 3 fold respectively, as compared to baseline (day 1) when measured 1 day after the last (10th) G-CSF injection. By 72 hours after each G-CSF dose, the number of leukocytes, neutrophils, and monocytes returned to normal [9].
The Vascular Surgery literature identifies the vicissitudes of standard of care management of CLTI, which includes invasive revascularization by bypass surgery, thrombo-endarterectomy, and catheter- based interventions (angioplasty, stent, atherectomy etc.). These procedures have significant risks and complication rates. Their high cost is compounded by the revisions required due to manage the variable and often limited durability of each intervention. There is an urgent need for a durable, lower risk, non-invasive, home based and inexpensive approach. The data presented herein supports further investigation into a novel cell therapy solution.
We achieved limb salvage with one month of filgastim and daily PCP until symptoms plateau in the worst of the CLTI population (“no-option” due to failed previous interventions or contraindications to such procedures). CLTI typically started to improve in the second week, and resolved by 5-6 months, at which time the PCP was stopped. The longest “no option” patient that achieved limb salvage was treated in 2008 and remains amputation free. Improvement in Ankle brachial index by an average of 49% was remarkably uniform and long lasting. While detailed review of the clinical experience is not in the scope of this article, obstacles we encountered to limb salvage included recurrent trauma, infection, poor compliance with wound management, and poor nutrition. There is a race between the rate NV and fibrinolyses were occurring and the rate of progression of the destructive effect of severe tissue ischemia. It is clear that improving the circulation is only one part of limb salvage, though it is arguably the most important. As with all vascular interventions, the earlier the intervention the better. The data sets presented in this manuscript support the need for a careful clinical assessment of this therapy on a broader scale [10].
G-CSF and fibrinolysis
The fibrinolytic cascade is quiescent in vessels that are chronically occluded. Chronic thrombus is relatively resistant to catheter directed fibrinolysis, requiring more than the safe 48 to 72 hour limit of intra-arterial infusion (due to risk of hemorrhagic complications). Furthermore, thrombus in the microcirculation (small arteries, arterioles, capillaries, venules) is relatively inaccessible during catheter directed thrombolysis of the larger thrombosed arteries within this safe 48-72 hour interval. Much of the infused thrombolytic agent (tissue plasmin activator) takes the path of least resistance and flows around these occluded areas [11].
G‐CSF was reported to stimulate activity of plasminogen activator in both extracellular and intracellular milieus of endothelial cells obtained from bovine arteries in 1989. This effect was dependent on the concentration of G‐CSF added to the culture medium and on the treatment time. Analyses by fibrin and reverse fibrin autography revealed that activity of plasminogen activator was much more increased than that of its inhibitor in endothelial cells treated with G‐CSF. In 2006, in vitro experiments suggested G-CSF associated fibrinolysis was directly attributed to the increase in circulating neutrophils. Neutrophils release enzymes reported having both pro-and antiangiogenic effects. Neutrophil induced fibrinolysis would be of direct benefit in managing complications of ischemia.
Despite these observations, there has been no assessment of the fibrinolytic potential of G-CSF in man. The data presented herein establish the increase in serum plasmin and fibrin degradation products. They support the angiographic evidence of fibrinolysis. Our novel treatment takes advantage of prolonged fibrinolysis to enhance the NV capability of G-CSF. The more vessel thrombus is lysed, the greater the endothelial shear stress caudally to incite arteriogenesis, and the greater the delivery of oxygenated nutritive blood flow as well as the clearance of toxic metabolic by-products. Our novel Filgrastim dosing interval safely induces natural fibrinolysis that lasts a month. This discovery introduces a new modality to approach the significant problem of chronic thrombus.
NV using G-CSF
NV by G-CSF was first described in 1989 when G-CSF and GMCSF were reported to induce endothelial cells to express an activation/differentiation program (including proliferation and migration) related to angiogenesis. Many NV clinical trials have been performed since with G-CSF. Results in the coronary and lower extremity, circulations have been mixed and have not changed standard of care. We believe one major cause for this is the duration of G-CSF administration in these trials, which is too short to effect fibrinolysis or NV. The extended dosimetry proposed herein has the potential to improve outcomes. Our therapy is presently one month long, not 5-10 days long. Our interval between doses is 72 hours, not 24 hours. The longer interval is sufficient to lower the intensity of the G-CSF effect, making treatment potentially safer, while at the same time prolonging the interval during which lysis and NV are promoted. Moreover, in the case of CLTI, the absence of PCP leaves the first 5 obstacles to NV (identified in the introduction) unaddressed (Table 5) [12].
| t=0 | t=2 hours | % Change (SD) | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Nitrite (mM) | Nitrite (mM) | ||||||||
| N | Mean | SD | N | Mean | SD | ||||
| PCP | Day 1 | 27 | 414 | 249 | 27 | 498 | 221 | 32(27) | <0.001 |
| Day 30 | 22 | 950 | 268 | 23 | 991 | 271 | 7(6) | <0.001 | |
| PCP and GCSF |
Day 1 | 8 | 401 | 232 | 8 | 490 | 222 | 28(28) | <0.001 |
| Day 30 | 4 | 1129 | 271 | 4 | 1199 | 372 | 7(4) | <0.001 | |
Table 5: Data from university of Chicago.
VEGF-A and HGF are both increased significantly by Filgrastim in the CLTI population. Both of these molecules are associated with endothelial cell growth and migration, and are stimulants of arteriogenesis and angiogenesis. Moreover, we show that MMP-9 is increased significantly, a molecule that helps with progenitor cell mobilization from the marrow in response to GCSF, as well as with matrix degradation during arteriogenesis.
The statistically significant increase in PDGF AA and BB caused by G-CSF supports NV. PDGF has a significant role in blood vessel formation. It promotes participation of pericytes, vascular smooth muscle cells, mesenchymal stem cells, and fibroblasts in vessel growth. These cells support the endothelial tube by providing vascular wall strength, impermeability and compliance [13].
PCP alone
Ischemic tissue is unhealthy. In the absence of oxygenated nutritive blood flow, oxidative phosphorylation becomes impaired, glycolysis is initiated, lactate is produced, and if there is insufficient local buffering the tissue pH drops. The latter can denature proteins, alter receptor activity and impair enzyme function. As ischemia progresses the tissue temperature drops, which affects the pKa of water and compounds protein dysfunction. Use of a PCP is an easy, low cost, home therapy solution to assist overcome impaired fluid mechanics in the extremity.
PCPs like the ArtAssist device used in this study are approved as a stand-alone limb salvage tool for a minimum of 3 hours a day in the seated position, particularly in patients who are not candidates for invasive limb revascularization, or who have had re-occlusion following previous such attempts. The amputation free survival data in “no option” CLI patients (N=62) treated with PCP alone at University of Chicago (UC) had an amputation free survival rate of 65% at 1 year, and 55% at 3 years, which approximates previous published experience. Serial hemodynamic testing in the UC experience showed a nonstatistically significant 9% rise (p=0.5) in ABI after 3 hours of PCP measured at 6 months. In contrast, a minimum PCP use of 8 hours a day (split into two 4 hour sessions) for 12 weeks, with repeat for another 12 weeks if symptoms persist, in 189 CLTI patients in Ireland did yield hemodynamic improvement during a mean follow up of 13 months. Toe pressures increased 15.5 mmHg (p<0.0001) and the mean popliteal artery flow increased from 35 to 56 cm/sec (p<0.0001); the ankle brachial index improved in non-diabetics only. Limb salvage at 3.5 years was reported to be 94% [14]. In contrast our combination therapy (PCP and G-CSF) increases the ABI 49% (p<0.004) measured at 6 months (2013-5 UC study) with a minimum of just 3 hours of daily PCP use, and has clear angiographic evidence of arteriogenesis and fibrinolysis.
Our chief biochemical measure of PCP efficacy was indirect measurement of endothelial activation, the key to initiating arteriogenesis. Rapid compression induces high shear stress. Shear stress in vitro has been reported to promote endothelial cell release of Nitric Oxide (NO). We measured this indirectly by measuring serum nitrite. We also measured an increase in MCP-1, a protein that is presented to the endothelial surface in the glycocalyx, and acts as a homing signal for circulatory monocytes to adhere to the endothelial surface. Once attached, these cells are dragged sub-endothelial where they assume macrophage like properties and begin the process of vascular remodeling. In our experiments with PCP alone we did not identify evidence of increased endogenous tissue plasminogen activator activity or of fibrinolysis. Lastly, one reported benefit of PCP use is that shear stress increases production of granulocyte-macrophage colony-stimulating factor by endothelial cells. We did measure GM-CSF by ELISA in the UC datasets, but the concentrations were too small, precluding meaningful analysis in our limited patient enrollment.
A disadvantage of cell therapies as well as PCP use is the gradual onset of efficacy. While our therapy yields pain relief within 10 days of onset of treatment, the ABI increase is noted after 4 to 5 more months of daily PCP use following the one month of GCSF and PCP together. Invasive revascularization on the other hand produces immediate results, albeit with the increased risk of complications and variable durability. Patients treated so far have anatomic alterations from previous intervention failures. The results of PCP and filgrastim should be even more appealing as a first line therapy in the absence of this added complexity.
We have not yet explored extending the administration of the GCSF to more than a month, or using pegalated G-CSF, and/or increasing the hours of daily PCP use. We also have not explored potential adjuvant therapies (e.g. growth hormone or other anabolic agents). Our overall goal is to promote NV as a low-risk durable first line home based treatment. If invasive revascularization should subsequently become necessary, at least the benefit of our therapy on the outflow could help improve its durability.
This is the first laboratory analysis of fibrinolysis arising during the use of filgrastim to promote neovascularization in advanced vascular disease (CLTI). The plasmin and fibrin degradation products data come from 2 independent laboratories and are highly significant. This brings management of tissue level microcirculatory thrombus within reach, without the risks of hemorrhage associated with prolonged intra-arterial fibrinolytic strategies used today. Additionally, in combination with protein evidence of neovascularization, our hypothesis that neovascularization and safe physiologic fibrinolysis can be orchestrated together simply by improving the ischemic tissue environment remains reasonable. The previous clinical trials using G-CSF in the lower extremity circulation will need to be revisited. We recommend adding PCP in the CLTI trials to activate the endothelium and improve the local environment. We also recommend prolonging the G-CSF effect to induce physiologic fibrinolysis for a sufficient amount of time. Trials of Filgrastim for the coronary and pulmonary circulations, and perhaps the cerebral circulation (lacunar infarcts from thrombosed lenticulostriate vessels) will need to be revisited at a dosimetry that capitalizes on both fibrinolysis and NV.
This study was funded by the 2016 cures within reach award, the university of Illinois at Chicago department of surgery, chaired by Enrico Benedetti, and the Warren Cole society. Rachana patil and yuanfan hong performed the assays under the supervision of Amelia Bartholomew at UIC. Patients were recruited by george havelka. The prior work was conducted starting in 2008 at the university of Miami and the Miami cardiac and vascular institute, and subsequently at the University of Chicago (UC) by Darwin Eton MD FACS DFSVS. Cathy Pabst RN coordinated patient care, along with at the Weiss Memorial Hospital where the first 8 patients from whom biomedical data were obtained were managed. ACI Medical Inc provided funding for assays, and provided the ArtAssist devices for all patients.
ACI Medical Inc. partially funded the study. All other authors declare no competing conflicts of interest.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Eton D, Bartholomew A, Zhou G, He TC (2023) Proteomic Evidence for G-CSF Induced Fibrinolysis and Neovascularization. J Cell Sci Therapy. 14:381.
Received: 17-Aug-2020, Manuscript No. JCEST-20-6071; Editor assigned: 20-Aug-2020, Pre QC No. JCEST-20-6071(PQ); Reviewed: 03-Sep-2020, QC No. JCEST-20-6071; Revised: 31-Mar-2023, Manuscript No. JCEST-20-6071(R); Published: 28-Apr-2023 , DOI: 10.35248/2157-7013.23.14.381
Copyright: © 2023 Eton D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.