Journal of Chemical Engineering & Process Technology
Open Access
ISSN: 2157-7048
ISSN: 2157-7048
Commentry - (2021)Volume 12, Issue 7
Energy is the key source of input to drive and improve the life cycle. The finiteness of fossil fuel has increased the demand for other sources. Biodiesels are promising alternative fuel and they are renewable. It has gained attention due to the smallness of fuels and environmental concern. The usage of liquid fuels prepared from used edible oil by transesterification process is one of the alternate methods for the use of fossil fuels. The recent focus relies on using used edible oil for producing biodiesel.
Biodiesel; Transesterification; Waste edible oil; Properties analysis
As population continues to increase, meeting the various energy needs such as domestic, industrial and transportation demands has become an increasing concern for policy makers and governments [1-3]. The economics of industrial societies is based on consumption of geological oil. These oils supplies are substantially concentrated in areas of political instability. It is expected that oil prices will increase significantly in day to day life. Biodiesel is usually produced from the vegetable oil or animal fat with short chain alcohol such as methanol or ethanol. Its use in diesel based vehicle has shown reductions in Pollutants. Burning of vegetable oil based fuel does not give net atmospheric CO2 level (Figure 1).
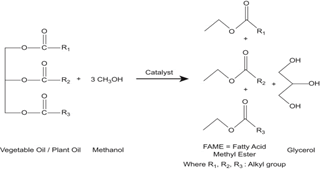
Figure 1: Tranâ??s esterification process.
This is the reaction to make our own biodiesel fuel from used cooking oil. We need is common chemicals and some equipments. The result is a cheap and clean burning, non-toxic diesel motor fuel
Requirements
Waste vegetable oil (WVO), Methanol (CH3OH), Hydroxide pellets (Figure 2) (Table 1)
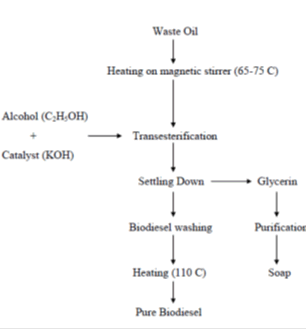
Figure 2: Production of biofuel from used edible oil.
| Property | Units | Value |
| Density | Kg/M3 | 910-924 |
| Kinematic viscosity | cst | 36.5-42.1 |
| Saponification value | Mg KOH/g | 186.1-208.3 |
| Acid value | Mg KOH/g | 1.35-3.56 |
| Iodine number | gI2/100Â g | 83-141.7 |
Table 1: Main Properties of Edible Oil.
Biodiesel has been prepared from various edible oil and it is found that it resembles the properties of commercial diesel produced from crude oil [4] (Table 2). The calorific values of the biodiesel are found within the range as per ASTM data. The Biodiesel from rice bran has the higher value of calorific value next to that of Jatropha oil [5].
| Properties | Diesel | Mahua oil | Rice bran oil | Jatropha oil | Sunflower oil | Used sunflower oil |
|---|---|---|---|---|---|---|
| Density (kg/m3) | 800-860 | 875 | 869 | 869 | 872 | 845.7 |
| Flashpoint (°C) | 65 | 170 | 175 | 165 | 166 | 164 |
| Specific gravity | 0.86 | 0.875 | 0.869 | 0.869 | 0.872 | 0.845 |
| Fire point °c | 72 | 178 | 178 | 172 | 177 | 174 |
| Calorific value (kcal/kg k) | 11164.8 | 10844 | 11185.4 | 11164.8 | 10844 | 10660 |
Table 2: Properties of bio-diesel from various oils.
Biodiesel generated from oil has a potential to become fuel of the future, of our country and it will not only provide employment opportunities in rural areas but also it provide an energy security, cleaner air and saving foreign exchange of our country. A variety of programs and solutions has been suggested to help alleviative energy needs. Since non renewable energy sources of energy are not going to last forever. The minimum reaction time required for maximum bio diesel yield was found to be 60 min–90 min. In the optimum conditions, the properties of bio diesel from rice bran oil, sunflower oil and used sunflower oil were compared. It has many properties similar to fossil fuel, which would allow modern diesel engines to use biodiesel without any major modifications to the engine, without any appreciable loss of engine performance.
Received: 12-Jul-2021 Accepted: 26-Jul-2021 Published: 02-Aug-2021 , DOI: 10.35248/2157-7048.21.12.423
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.