Journal of Developing Drugs
Open Access
ISSN: 2329-6631
ISSN: 2329-6631
Research Article - (2018) Volume 7, Issue 1
Received Date: Jan 30, 2018 / Accepted Date: Feb 14, 2018 / Published Date: Feb 22, 2018
The aim of this study was to prepare Paracetamol loaded Eudragit S100 nanoparticles by salting out (SO) technique. Eudragit S100 (ED) was used as a polymer. Paracetamol and polymer were dissolved in ethanol at various drug-polymer ratios (1:1, 1:2 and 1:3), among three formulations 1:3 was found to be the best formulation with drug content of 80.3% and entrapment efficiency was found to be 99.8%. Loading capacity was found to be more for 1:3 formulation. Na.cmc was used as stabilizer and ZnSO4.7H2O was used as a salting out agent and ethanol were used as solvent.
<Keywords: Salting out; Eudragit S100; Paracetamol nanoparticles (PCNPs)
Definition
Nanoparticles are sub- Nano sized colloidal drug delivery systems and the particle size ranges from 10-1000 nm in diameter. They are composed of synthetic or semi synthetic polymers carrying drugs or proteinaceous substances. Drugs are entrapped in the polymer matrix particulates or solid solutions or may be bound to particle surface by physical adsorption or in chemical form.
The selection of the appropriate method for the preparation of the nanoparticles depends on the physiochemical characteristics of the polymer and the drug to be loaded.
Advantages of nanoparticles
Nanoparticle can be administered by parenteral, oral, nasal, ocular routes. By attaching specific ligands on to their surfaces nanoparticles can be used for directing the drugs to specific target cells. Improves stability and therapeutics index and reduce toxic effects. Both active and passive targeting can be achieved by manipulating the particle size and surface characteristics of nanoparticles. Avoids hepatic first pass metabolism [1,2].
Disadvantages of nanoparticles
Small size and large surface area can lead to particle aggregation. Physical handling of nanoparticles is difficult in liquid and dry forms. Limited drug loading. Toxic metabolites may form [3].
• Cross linking methods: 1) By Cross-linking of Amphiphilic Macromolecules 2) By Crosslinking in W/O Emulsion 3) By Emulsion chemical dehydration 4) By Phase Separation 5) By pH induced Aggregation
• Polymerization methods: 1) Emulsion polymerization 2) Dispersion polymerization
• Polymer precipitation methods: 1) salting out method 2) solvent displacement (Nano precipitation) 3) solvent evaporation method [1].
Paracetamol also known as acetaminophen, is a medication used to treat pain and fever. It is typically used for mild to moderate pain relief. Evidence for its use to relieve fever in children is mixed. It is often sold in combination with other ingredients such as in many cold medications. In combination with opioid pain medication, paracetamol is also used for more severe pain such as cancer pain and pain after surgery. It is typically used either by mouth or rectally but is also available intravenously.
Effects last between two and four hours. Paracetamol is generally safe at recommended doses. Serious skin rashes may rarely occur, and too high a dose can result in liver failure. It appears to be safe during pregnancy and when breastfeeding in those with liver disease, it may still be used, but in lower doses. Paracetamol is classified as a mild analgesic. It does not have significant anti-inflammatory activity and how it works is not entirely clear. Paracetamol was discovered in 1877.
It is the most commonly used medication for pain and fever in both the United States and Europe. It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system. Paracetamol is available as a generic medication with trade names including Tylenol and Panadol among others.
Acetaminophen is thought to act primarily in the CNS, increasing the pain threshold by inhibiting both isoforms of cyclooxygenase, COX-1, COX-2, and COX-3 enzymes involved in prostaglandin (PG) synthesis. Unlike NSAIDs, acetaminophen does not inhibit cyclooxygenase in peripheral tissues and thus, has no peripheral antiinflammatory affects. While aspirin acts as an irreversible inhibitor of COX and directly blocks the enzyme's active site, studies have found that acetaminophen indirectly blocks COX, and that this blockade is ineffective in the presence of peroxides. This might explain why acetaminophen is effective in the central nervous system and in endothelial cells but not in platelets and immune cells which have high levels of peroxides. Studies also report data suggesting that acetaminophen selectively blocks a variant of the COX enzyme that is different from the known variants COX-1 and COX-2. This enzyme is now referred to as COX-3. Its exact mechanism of action is still poorly understood, but future research may provide further insight into how it works. The antipyretic properties of acetaminophen are likely due to direct effects on the heat-regulating centers of the hypothalamus resulting in peripheral vasodilation, sweating and hence heat dissipation [4].
EUDRAGIT polymers are copolymers derived from esters of acrylic and meth acrylic acid whose physicochemical properties are determined by functional groups. Eudragit S100 is commonly used for the formulation of controlled and sustained release dosage forms [5,6].
Materials
Drug: Paracetamol, Polymer: Eudragit S100, Solvent: Ethanol.
Paracetamol loaded Eudragit S100 nanoparticles were prepared by Salting out technique. Eudragit S-100 polymer was selected for this technique. Ethanol was used as a solvent.
Salting out method
Paracetamol loaded Eudragit S100 nanoparticles were prepared by salting out technique using ethanol as a solvent. Drug and polymer were dissolved in ethanol at various drug-polymer ratios (1:1, 1:2 and 1:3). Then the organic dispersion was added drop wise to the aqueous phase containing 2.5 gm of Na.CMC in 10 ml distilled water and 4.1 gm of ZnSO4·7H2O in 50 ml of distilled water. The stirring of O/W emulsion was continued for 3 hours. After 3 hours excess of distilled water was added to the formed dispersion. Then stirring was continued for 3 hours. The resultant dispersion was subjected for vacuum filtration. The dried product was subjected for 3-time washing with distilled water [7].
Evaluation studies: The obtained formulations of paracetamol loaded Eudragit S100 nanoparticle by salting out technique are evaluated for the following parameters.
Determination of drug content
Free drug of the formulations was first determined in the supernatant by choosing a solvent in which only the free drug gets dissolved and not the other ingredients. To determine the drug content, 50 mg drug equivalent to formulation was weighed accurately and transferred into 100 ml beaker containing 50 ml of methanol. The solution was stirred at 700 rpm for 3 hours by using magnetic stirrer. The resultant solution was filtered and the amount of the drug in the filtrate was estimated after suitable dilution by UV spectrophotometer at 257 nm [6].
Entrapment efficiency
Entrapment efficiency indicates the amount of drug encapsulated in the formulation. The method of choice for entrapment efficiency determination is separation of free drug by ultra-centrifugation, followed by quantitative analysis of the drug from the formulation. The samples were centrifuged by using ultracentrifuge at 17000 rpm for 40 min [7].
Percentage entrapment efficiency may be calculated from the following formula:
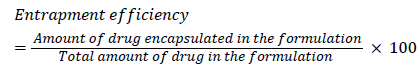
Loading capacity
It indicates the capacity of the polymer to load a drug.
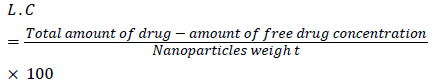
In vitro drug release kinetics
In vitro drug release studies were conducted by means of orbitary shaker. 50 mg of each accurately weighed formulation was transferred into 250 ml conical flask containing 50 ml pH 7.4 phosphate buffer. They were kept in an orbitary shaker at 100 rpm maintained at 37°C. Aliquots of 2 ml buffer was withdrawn at predefined time intervals and the medium was replaced with same volume of buffer. This study was carried out for 12 hours, and the amount of drug release was estimated by determining the absorbance at 257 nm using Elico UV spectrophotometer [8,9].
The obtained formulations were evaluated for the above-mentioned parameters and the results are discussed as follows.
Evaluation of paracetamol loaded Eudragit S100 nanoparticles by salting out technique
Paracetamol loaded Eudragit S100 nanoparticles were formulated at various drug-polymer ratios (1:1, 1:2 and 1:3).
Evaluation studies of PCNPS
Product yield: The dried nanoparticles were weighed and calculated for product yield, it was found that among the three formulations product yield was found to be best for 1:2 formulation.
Drug content: The drug content of all three (1:1, 1:2 and 1:3) formulations was evaluated.
From the Figure 1, 1:3 formulation showed maximum drug content 80.3% compared to 1:1 and 1:2 formulation (33.3 and 63.8%).
Entrapment efficiency: The entrapment efficiencies of 1:1, 1:2 and 1:3 were shown in Figure 2. On comparison, it was found that 1:3 formulation showed better entrapment efficiency i.e., 99.8% than 1:1 and 1:2 formulation [10,11].
Loading capacity: It indicates the capacity of the polymer to load a drug. From the results it was found that among the three formulations, 1:3 formulation showed highest loading capacity [12,13].
In vitro drug release studies: The drug release studies of all formulations of PCNPs were conducted by means of orbitary shaker for a time period of 12 hours. From the drug release studies as depicted in Figure 3, the results showed that 1:3 formulation showed maximum drug release rate of 28.31% within 12 hours. Whereas 1:1 and 1:2 formulation showed only 21.4% and 27.21% drug release rate [14].
For salting out technique
In this present study paracetamol loaded Eudragit S100 nanoparticles were prepared by salting out method by using ED as polymers, Na.CMC as stabilizer and Zinc sulfate as salting out agent. Each of three formulations were prepared by varying the concentrations of drug and polymer (1:1, 1:2 and 1:3). Among all formulations 1:3 formulation show better drug content and entrapment efficiency [15].
In the present study paracetamol nanoparticles were prepared by salting out technique. Among all the three formulations 1:3 formulation show better drug content and entrapment efficiency.
The authors sincerely thank Dr. M. Sumakanth, The Principal, RBVRR Women’s college of Pharmacy for providing access for library and databases. We also thank Mrs. Suvarna and Mrs. Sumalatha, for providing technical assistance.