Gynecology & Obstetrics
Open Access
ISSN: 2161-0932
ISSN: 2161-0932
Research - (2020)Volume 10, Issue 1
Background: During controlled ovarian hyper-stimulation COH, level of Progesterone on a day of human chorionic gonadotrophin (hCG) trigger for a final ovum maturation has a precious role for implantation of the embryos.
Aims: This study aims to investigate the incidence and the effect of Premature Progesteron Rise (PPR) on pregnancy rates PR as well as live birth rate LBR in ICSI cycle.
Material and Methods: A total of 710 patients undergoing ICSI cycles with fresh embryo transfer at Misurata National Infertility Centre were retrospectively involved. Patients included in the study who are <40 years with good ovarian response and had long agonist/antagonist ICSI protocols with fresh embryo transfer.
Results: The average age of patients was (32.26 years ± 4.924). The cut-off value found in this study to define PPR is (1.064 ng/ml). The overall incidence of serum PPR on hCG administration was 31.32%. The average serum PPR on a day of hCG administration was (1.1 ±1.8) ng/ml. PPR had a negative significant effect on PR (P-value 0.03).
Conclusion: In order to improve ICSI outcome, the PR is significantly negatively correlated with PPR. So, when serum P4 level is approaching the cutoff value (1.064 ng/ml) during COH, modification of the clinical treatment might be considered to improve the ICSI outcomes.
Premature progesterone rise; Clinical pregnancy rate; Ovarian cells
Pregnancy outcomes during Intra-Cytoplasmic Sperm Injection (ICSI) cycles are influenced by various physiological events secondary to COH. Progesterone is one of the important steroid hormones secreted under the effect of Follicle-Stimulating Hormone (FSH) from ovarian cells (granulosa and theca cells) and has a precious role in preparing the endometrium for implantation [1].
During controlled ovarian hyper-stimulation, the level of Progesterone on a day of human Chorionic Gonadotrophin (hCG) trigger for a final ovum maturation has a precious role for implantation of the embryos. In normal physiology, the level of estrogen increases during the follicular phase in response to the growth of recruited follicles. When the dominant follicles achieved, Luteinizing Hormone (LH) receptors will be expressed and this will lead to the secretion of progesterone in the different amount [2,3].
Premature Progesterone Rise (PPR) is can be described by a rise in a serum progesterone level on a day of ovulation trigger above a threshold level [4,5]. The pathogenesis of PPR is still under research and not well understood. However; there are many hypotheses explaining this pathology especially during controlled ovarian hyper-stimulation. Some of these proposed hypotheses are related to the rapid rising in estradiol level as well as insufficient pituitary suppression and that will increase the LH receptor sensitivity which is able to enhance granulosa cells to secrete progesterone [6].
Moreover, endometrial receptivity is an important factor for embryo implantation. It found to be dependent on many factors mainly; the progesterone exposure after sufficient estrogen exposition which will display morphological changes on the endometrium. Out of phase can be described by the presence of endometrial biopsy with a difference of more than 2 days between the histologic and the actual day after ovulation. No pregnancy could be achieved in patients with an “out of phase” endometrium which explained by the fact that endometrial advancement leads to an asynchrony between the endometrium and the developing embryo [7].
PPR (different threshold level) has been addressed as one of the negative factors affecting ART outcomes [8]. However; the conclusion around this subject is still not achieved as some studies state that no significant effect on implantation nor clinical pregnancy rate [1,4,8]. The objective of this study was to investigate the incidence and the effect of PPR clinical pregnancy rates and oocyte quality in ICSI cycles.
This is a retrospective study conducted at Misurata National Infertility Centre, Libya. 700 patients with different cycle protocols were included in the study. All etiologies of infertility were considered: tubal, male, idiopathic or associated with endometriosis. The inclusion criteria involved, all ICSI cycles with fresh embryo transfer, the age less than 40 years and have a good ovarian marker (FSH<10, AMH>1, AFC>5).
The data included in the study were hormonal blood tests on the 2nd and the 3rd day of the cycle involves estradiol, FSH, LH and AMH, as well as AFC by ultra-sound scan and plasma level of progesterone in the 36 h preceding ovulation induction with estradiol level results were recorded. In addition, oocyte maturation, pregnancy test and outcome had been recorded.
The protocols for ovarian stimulation according to standard protocols: long agonists or antagonists. The doses of gonadotropins used varied from 75 to 450 IU/day of stimulation (International Unity). These doses were various according to age, ovarian reserve markers and history of past stimulations. Ovulation triggering was depending on certain criteria such as at least two follicles over 17 mm and the plasma estradiol level. Oocyte retrieval took place 36 h after ovulation induction with hCG and embryo transfer was performed on day 2 or day 3 or blastocyst.
In this study, Progesterone assays were carried out on autoanalyser Cobas (Roche, Mannheim), the detection was made by Electro-Chemiluminescence.
The AMH measurement was done by the immunoassay supplied by Diagnostics Systems Laboratories (DSL) (a Roche Hitachi). FSH was measurement by Electro Chemiluminescence Immuno using (Beckman Coulter Inc). The AFC and normal anatomy of pelvic organs were determined by performing Trans-Vaginal Ultra-Sonography (TVS).
Patients received luteal phase support after oocytes retrieval by vaginal progesterone. Clinical pregnancy was defined by the presence of a gestational sac on transvaginal ultrasound at 7 weeks of amenorrhea.
Data were analyzed by software SPSS 23.0. Descriptive statistics were presented in terms of mean ± standard deviation for continuous variables. Whereas, the categorical data are presented by counts (percentage).
The student's t-test was used for comparison of the various parametric data. Receiver Operating Characteristics (ROC) analysis was performed to determine the cut‑off value for P4 at an approximately equivalent sensitivity and specificity, according to the pregnancy test result. The value (p<0.05) was considered to be statistically significant.
From 710, a total of 629 ovarian stimulation cycles (ICSI) were included in the study. There were 81 cycles were canceled either because of canceled embryo transfer or found already ovulated on a day of ovum retrieve and shifted to Intra-Uterine Sperm Injection (IUI).
The overall incidence of PPR is (197/629) 31.32%. The average patient's age was 32.26 ± 4.924 years. Regarding ovarian reserve markers, the averages were for FSH, AMH and AFC 7.4 ± 3.2, 3.7 ± 3.4, 9.4 ± 5.1 respectively.
The overall level of progesterone on a day of HCG trigger was (1.1 ± 1.8). All the population characteristics are shown in Table 1.
| Characteristic | Value |
|---|---|
| Age (year) | 32.2 ± 4.9 |
| Hormonal profile | |
| FSH (mIU/ml) | 7.4 ± 3.2 |
| LH (mIU/ml) | 5.9 ± 4.3 |
| E2 (pg/ml) | 39.4 ± 17.7 |
| AMH* (ng/ml) | 3.7 ± 3.4 |
| 8P4 (ng/ml) | 1.1 ± 1.8 |
| AFC | 9.4 ± 5.1 |
The values are presented either as mean ± SD or number (%)
Rolled on 454 patients of the population study*
FSH: Follicle Stimulating Hormone; LH: (Luteinizing Hormone); E2: Estradiol (on day 2 of the cycle); AMH: Anti-Müllerian Hormone; P4: Progesterone (on a day of hCG trigger)
Table 1: General characteristics of the study population.
Estradiol rates on a day of hCG in patients with elevated progesterone (P4>1.064 ng/ml) and the other group (P4 ≤ 1.064 ng/ml) were significantly higher: 2255.5 pg/ml, 1560.8 pg/ml respectively (P=0.02).
In addition, there was not found any significant relation between the two levels of progesterone in association with oocyte maturation, days of stimulation needed, No of oocytes retrieved, oocyte maturation and blastocyst transfer.
With regards to the protocols used in this study were antagonist and long agonist and most commonly used in the two groups (P4 ≤ 1.064 ng/ml, P4>1.064 ng/ml) was antagonist (69.68%, 72.08%) respectively with no statistically significant between them (Table 2).
| P4 ≤ 1.064 ng/ml | P4>1.064 ng/ml | P | |
|---|---|---|---|
| Stimulation duration | 9.01 ( ± 1.87) | 9.38 ( ± 1.75) | NS |
| E2 on hCG day (pg/ml) | 1560.8 ( ± 935.1) | 2255.5 ( ± 978.2) | 0.02 |
| No of oocytes retrieved | 8.75 ( ± 5.08) | 10.99 ( ± 7.34) | NS |
| Antagonist protocol | 69.68% | 72.08% | NS |
| Agonist protocol | 30.32% | 27.92% | NS |
| Mature oocytes | 59.49% | 54.31% | NS |
| Blastocyte transfer | 29.17% | 25.89% | NS |
Table 2: Serum progesterone levels on a day of hCG and different ICSI protocols, oocyte no. and maturity, estradiol level, and embryo transfer.
With regard to the pregnancy outcome, the group with P4 ≤ 1.064 ng/ml has a high percentage of positive pregnancy test 48.61% versus 16.24% for the other group P4>1.064 ng/ml and this has reported statistically significant correlation (P=0.039).
Moreover, Among pregnant cases in group with P4 ≤ 1.064 ng/ml, there were live birth rate (61.43%) and miscarriage rate (38.57%) vs LBR 40.63% and 59.37% for miscarriages for the other group P4>1.064 ng/ml with statistically significant correlation between the two groups (p=LBR 0.038 and Abortion 0.042). this is shown in Table 3.
| P4 ≤ 1.064 ng/ml | P4>1.064 ng/ml | P | |
|---|---|---|---|
| Pregnancy rate | 48.61% | 16.24% | 0.039 |
| Live birth rate | 61.43% | 40.63% | 0.038 |
| Abortion | 38.57% | 59.37% | 0.042 |
Table 3: Serum progesterone level on the day of ovulation trigger and the outcome of the ICSI and embryo transfer.
This study has used the area under the receiver operating characteristic curve (ROC-AUC) analysis which is an ideal method to detect the thresholds to determine the negative and positive cut‑off level for the predictor.
The area of the curve (from 0.9 to 1) represents excellent test, (0.8 to 0.9) good test, (0.7 to 0.8) fair test, (0.6 to 0.7) poor test, and (0.5 to 0.6) fail test. The ROC found the value for progesterone on a day of hCG to be 1.064 ng/ml for an equal (88%, 80%) level of sensitivity and specificity respectively (Figure 1).
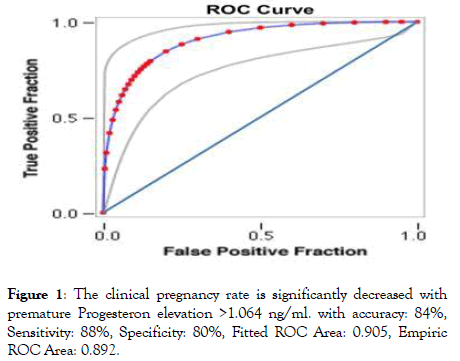
Figure 1: The clinical pregnancy rate is significantly decreased with premature Progesteron elevation >1.064 ng/ml. with accuracy: 84%, Sensitivity: 88%, Specificity: 80%, Fitted ROC Area: 0.905, Empiric ROC Area: 0.892.
It's clear that the progesterone level on a day of hCG trigger has a significant effect on pregnancy rate and ICSI outcome. Many studies have explained the high progesterone levels and its negative effect on the implantation of embryos which by advancement in the endometrial histological maturation as well as differentiation in its gene expression [9-11].
This study revealed a threshold level for progesterone on a day of HCG trigger 1.04 ng/ml and more will associated with a negative ICSI outcome in a term of pregnancy rate. On the other hand, elevated progesterone levels on a day of hCG have no impact on oocyte quality nor live birth rate.
In the literature, Venetis et al. in their meta-analysis revealed that PPR associated with low pregnancy in women undergoing fresh ICSI cycles, with a threshold (0.8-1.1 ng/ml). Also, Singh et al. have studied the effect of PPR on ICSI outcome and used ROC analysis to identify the threshold cut‑off value for progesterone which was found to be 1.075 for an equivalent (53%) level of sensitivity and specificity and the result was significantly inversely correlated with pregnancy rate. Moreover, in the study by Mascarenhas et al., revealed the progesterone elevation was associated with a significant reduction in clinical pregnancy rate (P=0.0092) with the cutoff for PPR being ≥ 1.2 ng/ml. It is clear that these studies have shown results in agreement with this study's results and the same cut‑off values for progesterone level on a day of trigger [12-14].
There have been numerous studies that have evaluated the relation between progesterone elevation on a day of oocyte trigger and the pregnancy rate that ended with conflicting results. Venetis et al. Found progesterone thresholds of 0.9 ng/ml determined by ROC curves and revealed no significant association of elevated progesterone on pregnancy rates and this is against the results in this study. Nagaraja et al. revealed that the serum progesterone level estimated on the day of ovulation trigger was not statistically significant difference observed with the pregnancy rate. In addition, Julien et al. found that the progesterone elevation has no negative effect on pregnancy rate. However, it is negatively influenced by the live birth rate and an increase of miscarriage [1,4,8].
This study included all types of protocols with no significant difference in the result of negative effects of PPR and this was in consensus with studies by Ashmita et. al, found that Clinical pregnancy rates in relation to the PPR were significantly impaired in both agonist and antagonist protocols. However, Bosch et. al, found that the deleterious effect of PPR is higher in agonist protocol than antagonist and Huang et. al, revealed the same result but with a different P4 threshold level [3,4,15,16]. On the other hand, Nagaraja reported that the incidence of PPR did not differ with different stimulation protocols nor did it affect pregnancy rates [4].
With regards to the relation between Estradiol (E2) level and Progesterone level on a day of hCG, there was no significant relationship between them which is shown with previous studies by Kyrou, Singh and Wu Z [2,13,17]. However, the result of this study and other studies by Bosch and by Ashmita reported a significant rise in the incidence of PPR with a higher level of E2 (p-value 0.02, 0.0001, 0.006) respectively [3,12]. Many studies revealed a positive correlation between the number of mature follicles and PPR [13]. However, other Ashmita and Kyrou showed PPR significantly correlated with a higher number of immature oocytes that usually associated with failed fertilization or ended by abortion [2,3].
Thereafter, it is clear that there is a conflict between the previous research regarding the effect of PPR and pregnancy outcome. However, from a cost-effective point view, and prior published works; this study recommends that the progesterone thresholds viability level has to be determined and accordingly, various measures have to be taken in order to improve the ICSI outcome.
This study provides the correlation between premature progesterone elevation and pregnancy rate. The clinical pregnancy rate is significantly negatively correlated with PPR. It is recommended that, when serum P4 level is elevating and approaching the cutoff value (1.064 ng/ml) during control ovarian stimulation, modification of the clinical treatment should be considered to improve the ICSI outcomes.
Citation: Eljabu H, Elfortia I, Andisha A, Suliman S, Alrayes A, Elmahjoub E, et al. (2020) Pre-Mature Raise of Progesterone and its Effect on Clinical Pregnancy Rate and Live Birth Rate in Intra-Cytoplasmic Sperm Injection (ICSI) Cycles. Gynecol Obstet (Sunnyvale)10: 516. doi: 10.35248/2161-10932.19.10.516
Received: 13-Jan-2020 Accepted: 20-Jan-2020 Published: 27-Jan-2020 , DOI: 10.35248/2161-10932.20.10.516
Copyright: © 2020 Eljabu H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.