Journal of Horticulture
Open Access
ISSN: 2376-0354
ISSN: 2376-0354
Review Article - (2022)Volume 9, Issue 1
Loquat (Eriobotrya japonica Lindl.) fruit is famous for its taste and rich source of phenolics, flavonoids, sugars, carotenoids and different acid contents. It is non-climacteric fruit grown under sub-tropical and tropical conditions. There are two distinct cultivars including white and yellow-flesh with variable fruit biochemical composition and growth pattern. Different preharvest factors like soil, climate, rootstock, fruit bagging, pruning, water application, nutritional management and mulching have distinct effect on fruit quality. There is series of physical, biochemical and physiological changes in fruit ripening. Sugar accumulation, phenolic and carotenoid biosynthesis, organic acid metabolism and different volatile compounds are main indicators of fruit harvest maturity. Ethylene biosynthesis and respiration patterns also contribute the developmental process. To increase fruit size and improved quality, many chemicals especially plant growth regulators have been applied. This review paper mainly covers the pre-harvest factors affecting fruit quality and metabolic dynamics of fruit ripening.
Eriobotrya japonica; Chemical application; Harvest maturity; Fruit maturation; Physiology
Loquat (Eriobotrya japonica Lindl.) is a non–climacteric and subtropical fruit that belongs to Rosaceae family. It was originated from the hills of Southeast China about 2000 years ago and later on introduced in Japan during Han Dynasty about 100 BC and then spread all over the world. It is commercially cultivated in Brazil, China, Cyprus, Greece, India, Israel, Japan, Pakistan, Spain, Turkey and USA (Table 1) [1].
| Country | Cultivar | Rootstock |
|---|---|---|
| China | 'Bahong', 'Baiyu', 'Changhong', 'Chuannao', 'Dahongpao', 'Guangrong', 'Hongmao', 'Jiefangzhong', 'Jinfeng', 'Liufenzhong', 'Louyangqing', 'Qingzhong', 'Tanaka', 'Thales', 'Zauhuang' | Loquat seedlings, Quince |
| Japan | 'Advance', 'Akko 1', 'Champagne', 'Early Red', 'Kusunoki', 'Mizuho', 'Mogi', 'Obusa', 'Togoshi', 'Tsukumo', 'Yehuda', | Loquat seedlings, Quince |
| India | 'Ahdar', 'Ahmar', 'Asfar', 'Fire Ball', 'Golden Yellow', 'Improved Pale Yellow', 'Large Agra', 'Large Round', 'Matchless', 'Safeda', 'Tanaka', 'Thames Pride' | Loquat seedlings |
| Pakistan | 'Safeda', 'Surkh', 'Tanaka', 'Golden Yellow' | Loquat seedlings |
| Spain | 'Magdal', 'Golden Nugget', 'Algeria', 'Tanaka' | Loquat seedlings, Quince |
| Turkey | 'Akko 13', 'Golden Nugget', 'Tanaka', 'Hatif Çukurgöbek' | Loquat seedlings |
| Egypt | 'Morphou', 'Karantoki', 'Golden Ziad', 'Maamora Golden Yellow', 'Premier', 'Early suckary', 'Large round', 'Advance', 'Late Victoria' | Loquat seedlings, Quince |
| Russia | 'Champagne', 'Comune', 'Grossa de Sicilia', 'Premier', 'Tanaka', 'Thales' | Quince |
| USA | 'Advance', 'Early Red', 'Eulalia', 'Champagne', 'Golden Red', 'Mammoth', 'Oliver', 'Pineapple', 'Premier', 'Tanaka', 'Thales', 'Victor', 'Wolfe', | Loquat seedlings |
| Brazil | 'Precoce de Itaquera', 'Mizuho' | Loquat seedlings |
| Cyprus | 'Morphou', 'Karantoki' | Loquat seedlings |
| Australia | 'Bessell Brown', 'Glenorie Superb', 'Mammoth', 'Swell's Enormity', 'Victory', | Quince |
| Israel | 'Akko 1', 'Akko 13', 'Saint Michel', 'Tsrifin 8' | Loquat seedlings |
| Greece | 'Rozenon', 'Troulotis', 'Koilarato' | Loquat seedlings |
| Italy | 'Nespolone di Trabia', 'Nespolone Bianco', 'Vainiglia', 'Sanfilippara', 'Virticchiara' | Loquat seedlings, Quince |
| Morocco | 'Tanaka', 'Saint Michel', 'Algerie' | Quince |
| Portugal | 'Tanaka', 'Algerie', 'Golden Nugget' | Loquat seedlings, Quince |
Table 1: Major cultivars of loquat grown in different countries.
China is the largest producer among all of them with total 460,000 tons annual production followed by Spain (45,000 tons), Turkey (12,000 tons), Pakistan (10,479 tons) and Japan (10,245 tons) [2]. The fruit is oval or round in shape with golden color. Although, there are many seedless cultivars being produced but typically fruit is pome like having many seeds [3]. On an average, there are three to five seeds in a single fruit that usually contain about 25%–35% of the total fruit weight [4]. Loquat is famous for its sweet and slightly acidic taste, rich in carotenoids, lipoproteins, Total Phenolic Contents (TPC), minerals and vitamins (Table 2) [5].
| Constituent | Content |
|---|---|
| Ash (g) | 0.4-0.5 |
| Calories (Kcal) | 47-168 |
| Carbohydrates | 9.6-43.3 |
| Total carotenoids (µg) | 196-3020 |
| Carotene (µg) | 559 |
| Calcium (mg) | 16-70 |
| Dietary fibers (g) | 0.8-1.7 |
| Fat (g) | 0.2-0.7 |
| Iron (mg) | 0.24-1.4 |
| Magnesium (mg) | 13 |
| Protein (g) | 0.43-1.4 |
| Phosphorous (mg) | 20-126 |
| Potassium (mg) | 266-1216 |
| Sodium (mg) | 1 |
| Total phenolics (mg) | 33.6 |
| Total flavonoids | 24.3 |
| Vitamin A (IU) | 1528-2340 |
| Vitamin C (mg) | 1.0-3.0 |
| Water (g) | 86.5-88.2 |
Table 2: Nutritive value of loquat fruit per 100 g fresh weight.
In addition, it also contains many bioactive compounds including triperpenic acid and flavonoids [6]. It is considered very effective against the oxidative low density lipoproteins as it shows high rate of radical scavenging activity in the human body [7,8]. Flavonoids are only present in the peel tissues of loquat fruit. Each loquat cultivar has different profile of TPC and ‘Mizauto’ shows the highest phenolic contents. Phenolic profile of six loquat cultivar viz ‘NE–3’, ‘Aria’, ‘Mizumo’, ‘Mizauto’, ‘Nectar de Cristal’ and ‘Mizuho’ was studied by Ferreres and found that 5 feruloylquinic and, 3 and 5 caffeoylquinic acids are the primary components in all cultivars (Figure 1) [9].
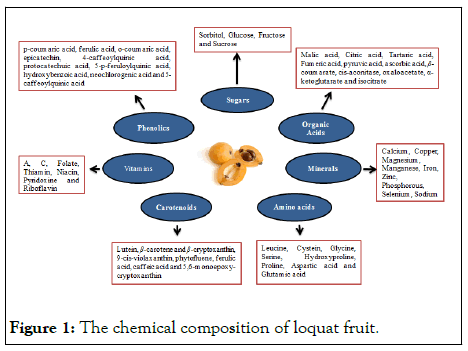
Figure 1: The chemical composition of loquat fruit.
Loquat fruit is orange to yellow in color at fully mature stage due to the presence of carotenoids. Lutein, β–carotene and β–cryptoxanthin are the major carotenoids in the loquat fruit flesh at the time of maturity [10]. It is also a good source of provitamin due to retinol activity equivalent that ranges from 80 to 162 μg per 100 g fresh weight. Flavor of fruit is directly correlated with the sugar to acid ratio [11]. Reported that the major sugars in the loquat fruit are sorbitol, glucose, sucrose and fructose. Ascorbic Acid (AsA), citric acid, fumeric acid, malic acid, pyruvic acid and tartaric acid are also present in the loquat fruit pulp. Malic acid is the major organic acid and contributes about 20%–60% of total organic acids in loquat fruit. As the level of reducing sugars decreases with the advancement in the storage period, sugar to acid ratio increases leading to the deterioration of fruit flavor.
The purpose of this paper is to review the preharvest factors affecting fruit growth and physiological changes during ripening and maturation of fruit.
Preharvest factors affecting fruit growth and quality
Soil and climate: Soil is one of the most important factors for the optimum growth of horticultural commodities. Loquat can be grown in a variety of soils including alkaline and acidic soils with good drainage; however, sandy loam is the best soil for its optimum growth. The soils having poor drainage produce low quality fruit with good flavor and color but with large seed size and small proportion of flesh [12]. Loquat is subtropical fruit crop that grows best in those areas where average temperature is 10°C–30°C. It can be grown up between latitude from 20° to 45° south and north [13]. However, it is highly sensitive to the frost and chilling temperature especially at flower bud initiation; so, it is well adapted to Mediterranean region with the mild temperature conditions [2]. It grows well in the partial shade or under direct sunlight as heat is necessary for the maturation of fruit; however, extreme heat during summer may cause leaf scorching and sunburn. For the optimum growth of fruit, well distributed rainfall ranging from 100 to 250 cm is required throughout the growing season [12].
Pruning and training: Pruning and training directly influence the fruit quality by increasing the light penetration and aeration to the trees that consequently leads to the higher production with improved quality. Production of plant dry matter content is directly correlated with the light penetration; however, the removal of diseased shoots by pruning is also beneficial for crop improvement [14]. Without proper pruning and training, loquat tree exhibits upright and excessive growth. Such conditions lower the labour efficiency during orchard management and plants are susceptible to the strong winds. Traditionally, there are two systems adopted by the Chinese growers including open center system and modified central leader system to allow maximum light penetration to the trees by pulling the branches down. There was significant increase in fruit set and quality was improved when loquat trees were pruned by open center system [15]. Training of the loquat trees in the vase shape is also very important. The best season for the pruning is late autumn or winter at the time of flower bud initiation. Sprouts and diseased branches should be trimmed on regular basis [16]. Reported that there was maximum yield and fruit quality when ‘Luoyangqing’ loquat was pruned during summer season one week after fruit harvest and trained in central ladder system. Therefore, pruning is key factor for the proper fruit growth in loquat. There was also the improved biochemical profile for those fruits that were ripened on the pruned trees.
Flower and fruit thinning: Loquat trees bloom profusely during autumn season on the terminal shoots. Primary panicles that sprout during spring season have better inflorescence; produce earlier and more vigorous fruits. Summer panicles sprout on lateral branches are weak and low yielding with fewer fruits [2]. For the proper fruit development and yield, thinning of fruits or flowers is necessary step. In loquat, thinning of flowers is done up to the extent that there should be ten fruits in each inflorescence. In Japan, fruit thinning is done to reduce the number of fruits in each bunch up to 4 fruits and this helps to increase the productivity and quality of the fruits [17]. Auxins are considered most suitable chemical for flower and fruit thinning in loquat [18]. Investigated the effect of NAA foliar application on ‘Golden Nugget’ and ‘Algerie’ loquat and observed that there was 20–65% decrease in fruit intensity and fruit diameter was increased up to 10% by 20 mg L–1 NAA treatments, than control. The color development in loquat was also improved by this application with considerable increase in TSS. Consequently, fruits mature earlier by NAA application [19]. Also reported that loquat fruit yield and quality was improved with NAA application. Therefore, due to long blooming period in loquat, the best time for fruit thinning in loquat is considered immediately after the cold period.
Bagging: This is the most effective and essential technique to protect the fruits during development with improved commercial properties, appearance and decrease in the disease incidence. It improves the microenvironment of fruits during developmental stages that lower organic acids and fruit drop [20]. This practice is carried out immediately after fruit thinning by using appropriate bagging material. Most of the times, polythene translucent bags are preferred but it may leads to the incidence of physiological disorders [15]. Old newspaper, paper bags and nets may also be used for bagging [21]. Found that there was considerable increase in soluble solids, glucose and sucrose contents with substantial decline in titratable acidity, sorbitol and fructose in bagged ‘Qingzhong’ loquat fruit with lower activities of Sucrose Phosphate Synthase (SPS) and Sucrose Synthase (SS) enzymes in non–bagged fruits; while, Neutral Invertase (NI) and Acid Invertase (AI) enzymes activities were considerably higher in non–bagged fruits. Sorbitol is an important component in the loquat fruit during fruit development and bagging considerably lowered the sorbitol metabolism by delaying the activities of SOX and SDH enzymes which clearly indicate that bagging increased the rate of photosynthesis in the fruits by increasing SPS and SS enzymes activities [22]. Proposed that there was positive correlation between bagging material transmittance and sugar content as sugar contents were higher in fruits bagged with high light transmitting material [23]. Also observed that bagged ‘Baiyu’ and ‘Ninghaibai’ loquat fruit exhibited excellent appearance with higher AsA and carotenoid contents. The quality of bagged fruit is directly linked with the type and quality of bagging material, such as shengda bags were the most effective bags that improved fruit quality in ‘Jiefangzhong’, ‘Baiyu’ and ‘Qingzhong’ loquat fruits [24-26].
Irrigation and mulching: Loquat is hardy plant which can tolerate water stress. There is no specific need of irrigation for loquat trees; however, irrigation at the time of fruit maturation is very important for the proper fruit development. In hot summer, sunburn is the main constraint that lowers the fruit quality and may be reduced by irrigation at regular intervals of two to four weeks [27]. Level of irrigation is directly correlated with the photosynthetic pigments in leaves; chlorophyll b content is more susceptible to damage in comparison to chlorophyll a [28]. In Asia, sod culture is very famous for loquat cultivation with two to three annual mowing and the grass trimmings are usually used as mulch for the crop improvement. Organic substrates and green manures are important source of nutrition for loquat in China. There was 2°C increase in soil when film mulch was applied in 10–13 years old loquat orchards in Zheijang. This thin film may be transparent, black or brown plastic sheet and may be applied from November to May [29]. Reported that there was significant improvement in soil bulk density, nutrient status and soil moisture with higher yield provided with the adequate soil NPK and mulching of polyethylene film.
Nutritional management: Application of fertilizer is critical index for the optimal growth of plants. Soil nutrient status and plant age are directly associated with the application rate and type of fertilizer used in loquat. The detailed study on the requirement of NPK in loquat was done by Sato and Fujisaki, who proposed that fertilizer application is very important in young juvenile loquat trees at the interval of two months. They further proposed that NPK application depends upon the soil fertility. On an average, orchard having mature bearing plants should be supplemented with 180, 130 and 140 kg per ha NPK, respectively. The application of the nutrients varies from area to area and the standard application of NPK in Japan is 170, 115 and 125 kg per ha, respectively, for ‘Mogi’ fruit trees. The whole fertilizer is to be applied in the form of split doses for optimum vegetative and reproductive growth. In addition to NPK, application of minor nutrients is also advantageous for the development of fruit. Application of calcium improves the fruit quality by lowering the incidence of pit disorder that is characterized by the blackening of fruit. Moreover, secondary nutrients like molybdenum, boron, zinc and manganese are also very crucial in fruit development and should be applied in 2–3 doses in a year. These minor nutrients should be applied in the form of foliar spray and best time is before the blooming season. In case of excess plant growth or yellowing of leave edges, fertilizer should not be applied during summer season. Deficiency of minor nutrients especially iron may be identified by the green veins and yellow leaves. This deficiency of minor nutrients is directly correlated with the alkaline nature of soil [30]. The availability of micronutrients to the plants is associated with the high pH and excess of phosphates in the soil; however, it may be improved by the application of sulfur under the tree canopy or by reducing the phosphorous application. Moreover, high pH may also be treated with iron chelates drenching. Organic manures may also be applied in loquat orchards preferably at late fall or before flower bud differentiation [2].
Cultivars and rootstock: There are two types of loquat cultivars including Chinese and Japanese loquats that can be differentiated on the basis of fruit color, presence of seeds in fruit and harvesting season. The Chinese group is relatively late maturing with good postharvest quality. The fruit is sub–acidic with less juicy content, numerous small size seeds and good flavor. The fruits have light orange skin and dark orange flesh with pear to round in shape. The Japanese group is relatively early maturing, long to oval shape and has less number of large seeds in the fruit [3]. All loquat cultivars may be divided in to two main groups on the basis of the flesh color; yellow–orange– and white–fleshed (Table 3).
| White flesh | Yellow-orange flesh |
|---|---|
| 'Advance' | 'Ahmar' |
| 'Ahdar' | 'Akko 13' |
| 'Asfar' | 'Big Jim' |
| Benlehr | 'Early Red' |
| 'Champagne' | 'Eulalia' |
| 'Fire Ball' | 'Golden Red' |
| 'Herd's Mammoth' | 'Golden Nugget' |
| 'Large Round' | 'Golden Zaid' |
| 'Pale Yellow' | 'Large Agra' |
| 'Pineapple' | 'Matchless' |
| 'Premier' | 'Mogi' |
| 'Safeda' | 'Mrs. Cooksey' |
| 'Saint Michel' | 'Obusa' |
| 'Victor' | 'Tanaka' |
| Vista White | 'Wolfe' |
Table 3: Most common white and yellow-orange-fleshed loquat cultivars.
Evaluated 54 different loquat cultivars and assessed their traits in China on the basis of apple SSR markers. They categorized these cultivars on the basis of their genetic homo or heterozygous characters and identified their flesh colors. The selection of rootstock is very important for the proper growth and development of fruit crops. Loquat is grown by seeds when it is to be used as rootstock. Loquat seedlings, pyracantha, quince, pear and apple are used as rootstock in different countries; however, loquat seedlings are preferred for this purpose on all other available rootstocks (Table 1). The fruiting time is 9–10 years for sexually propagated loquat; while, it took 4–5 years in vegetatively propagated trees for maiden bearing [31-32]. The plants propagated on pyracantha and quince rootstocks show dwarfing of plants which lower the labour consumption for training, fruit thinning and harvesting; but, those grafted on loquat seedlings leads to the taller trees. There is higher tolerance in quince against the wet and heavy soil but many suckers may be produced as the outgrowth [2].
Preharvest chemical application: Preharvest application of minerals and other chemical are very crucial for proper fruit development and may lead to the extended postharvest life with best quality [33]. Preharvest foliar application of micronutrients has been proved very important regarding storability and quality of fruits by decreasing the disease incidence and abiotic stresses [34]. Loquat fruit growth also increases when there is foliar application of micro nutrients that subsequently promote fruit maturation and improve fruit size. Postharvest quality of loquat depends upon the nutritional status during fruit maturation and there was higher TSS content with less weight loss during storage when ‘Improved Golden Yellow’ loquat fruit were sprayed with zinc sulphate and calcium nitrate immediately after fruit set. There was better organoleptic attributes in foliar sprayed fruit over those obtained from control trees [35]. Fruit firmness is the key indicator for loquat fruit quality and soft fruit will be more susceptible to mechanical injury and bruising during harvest and handling. The firmness of loquat fruit directly depends upon the lignin deposition and Ca content in peel tissues. Exogenous application of Ca improved the fruit firmness in ‘Algerie’ loquat when calcium chelate was applied after fruit set [36]. Plant Growth Regulators (PGR) are very important in plant metabolism and fruit development. They are crucial for proper growth and development with better biochemical and physiological aspects. Preharvest NAA application at bloom and young fruit stages significantly improved TSS, vitamin C and fruit weight with less TA content in ‘Jiefangzhong’ loquat fruit as compared to untreated control [37-38]. Proposed that there was better fruit quality and yield when GA3, IAA and Benzyl Amino Purine (BAP) were applied at bloom stage in ‘Soucary’ loquat fruit tree. There was less TSS, TA and AsA content in control fruits in comparison to PGR application fruits. Best results were presented by GA3 among treatments while minimum deviation was observed in BAP treated loquat fruits.
There are mainly two distinct phases in loquat fruit ripening; first is seed growth and second is maturation phase and it is characterized by softening of fruit pulp, color development and decrease in organic acid contents with considerable increase in fruit weight.
Respiration and ethylene production
Ethylene production in loquat in relation to the fruit ripening and maturation is very controversial as there are different assessments made by the different researchers. Some researchers found remarkable increase in respiration rate and ethylene production with the onset of color break stage in loquat fruit [39-40]. On the basis of these observations, loquat was classified as climacteric fruit [41]. Climacteric classification is not only based on sharp rise in the ethylene production but also the associated with increase in the respiration rate during the physiological maturity of the fruits. The controversy in the classification of the loquat as climacteric fruit is based on the observations that although there is a sharp increase in the ethylene production but no significant rise in the respiration rate at any stage of the fruit development as observed by Zheng [42]. These observations and reports made it very difficult to classify loquat as climacteric fruit because (1) no specific response was observed in the ripening of loquat fruit in relation to ethephon application (2) irregular pattern of CO2–C2H4 relationship in loquat fruit although it has been reported in many climacteric fruits (3) there is no evidence of pectin– disrupting, cellulose and hemicellulose degrading enzymes activities which are in close association with ethylene production (4) there is zero translocation of sugars during growth due to hydrolysis [43-47]. Studied the relationship of genes expression and ethylene biosynthesis at different ripening and developmental stages, and successfully cloned three genes including EjACO1, EjACO2 and EjACS1 from ‘Luoyanqing’ that are responsible for ethylene biosynthesis in loquat. The expression of EjACO2 was observed in petals and leaves; whereas, EjACO1 and EjACS1 were expressed in fruits. At color break stage, ethylene production was at peak and EjACO2 expression pattern was consistent with ethylene biosynthesis. All these genes exhibited low transcription level when stored at 20°C. Moreover, respiration rate and ethylene biosynthesis was increased during fruit growth and it was proposed that there was significant role of EjACO2 in this increase [48]. Investigated the respiration pattern of loquat throughout the fruit development in relation to CO2 release in ‘Centenaria’, ‘Mizauto’, ‘Nectar de Crystal’, ‘Mizumo’ and ‘Mizuho’ cultivars. ‘Centenaria’ expressed lowest CO2 release; while; maximum respiration rate was observed in ‘Mizumo’ and Mizauto’ 2 days after harvest. There was continuous increase in respiration rate throughout the fruit development while it continuously declined since 3 days after harvest in storage [49]. Observed increasing respiration rate till light green stage of fruit development and declined thereafter with regular decline in ethylene biosynthesis. There was no climacteric characteristic in ‘Algerie’ fruit including increase in ethylene biosynthesis and respiration rate at any stage during fruit maturation. Moreover, there was no increase in both these processes during postharvest storage and similar pattern was observed both in unripe and ripe fruit [11]. Also proposed similar results in ‘Mogi’ loquat fruit which clearly indicate that loquat is non–climacteric in nature.
Carotenoid biosynthesis
Color is most important indicator of maturity and ripening in loquat fruit, and its color is mainly due to carotenoids in pulp and peel tissues with yellow to red depending upon cultivar. The concentration of carotenoids increases with the increase in growth period due to the reduction in organic acids and increase in the sugar levels [50]. There is considerably higher carotenoid content in yellow–red fleshed loquat fruits in contrast to white fleshed cultivars. Similarly, significantly lower carotenoid content (91.52–202.28 μg/g DW) in white fleshed loquat cultivars as compared to yellow fleshed (214.50–475.22 μg/g DW) loquat cultivars were observed [26]. There was higher β– carotene in yellow fleshed fruits and there was also a characteristic deviation in caroteneoid content in pulp and peel tissues with lutein and β–carotene as major components making more than 60% of total carotenoids in yellow fleshed while β– carotene account more than half of total carotenoids in white fleshed loquat although 18 different types were identified in flesh and 32 in peel tissues [51]. There are less carotenoid with higher chlorophyll content in immature fruits which is being synthesized with the progress in fruit development and chlorophyll content decreases simultaneously, subsequent to fruit color change till fully ripened stage. Although there is higher carotenoids both in white and yellow fleshed fruit at immature green stage but there is higher synthesis and accumulation in yellow fleshed throughout the fruit development and reaches at highest level at mature stage in yellow over white fleshed loquat [52]. Major carotenoids accumulation site in plant cell is plastid that may be differentiated from chromoplast to chloroplast in ripening process [53]. Observed higher chromoplast with larger size in pulp and peel of yellow fleshed ‘Luoyangqing’ compared to white fleshed ‘Baisha’ loquat fruits but there was no chromoplast in pulp of ‘Baisha’ fruit. Transcription of ORANGE (OR) gene is major regulator for chromoplast differentiation and it was also evaluated that there was comparatively lower OR gene expression in ‘Baisha’ than ‘Luoyangqing’ which may be the cause of less carotenoid biosynthesis in white pulp loquat cultivars.
Transcription analysis of gene expression for carotenoids biosynthesis in loquat fruit development was assessed by many scientists to optimize and to understand the exact mechanism of its regulation and their deviation in white and yellow fleshed loquat. Gene expression for carotenoids biosynthesis in white fleshed loquat revealed that the lower carotenoids level in white fleshed cultivars is mainly due to suppression of β–carotene–3– hydroxylase gene (HYB). As the maturation period prolonged, there was down regulation of HYB gene expression that is mainly positively correlated with β–carotene biosynthesis, hence there is lower β–carotene in white fleshed fruits [54]. In contrary, there is up regulation of HYB gene expression in yellow–orange–red fleshed loquat fruits. Moreover, the lower carotenoid content in ‘Baisha’, a white fleshed cultivar, is low due to low expression of β–Carotene Hydroxylase (BCH), chromoplast–specific lycopene β–Cyclase (CYCB) and Phytoene Synthase 1 (PSY1) genes; while, higher expression of BCH, CYCB, PSY1 and ζ–carotene desaturase (ZDS) genes are correlated with higher carotenoid in yellow fleshed ‘Obusa’ fruits during fruit development [51]. Hence, the upregulation of carotenoid content during fruit development is directly associated with EjBCH, EjCYCB and EjPSY genes expression from which PSY is the most important gene for carotenoid biosynthesis [55-56]. Evaluated the gene expression pattern of four PSY genes in ‘Luoyangqing’ and ‘Baisha’ loquat and found that EjPSY1, EjPSY2A and EjPSY2B, were responsible for carotenoid biosynthesis in peel, fruit flesh and leaves, respectively; however, there was no specific role of and EjPSY3 in carotenoid biosynthesis. Another very important finding was the identification of mutant form of EjPSY2A identified as EjPSY2Ad in white fleshed fruits that was main reason for less carotenoid biosynthesis. The role of PSY in carotenoid synthesis was confirmed by Virus Induced Gene Silencing (VIGS) technique through the suppression of EjPSY gene and it was clearly indicated that there was green carotenoid biosynthesis in gene silenced fruits while there was normal yellow carotenoid synthesis in control fruits, hence the role of PSY gene was confirmed in loquat.
Sugars metabolism
Sugars are the integral part of fruit flavor and quality. Sucrose is the predominant among all other sugars in ripe loquat fruit which accumulates at faster rate at the beginning of maturation. On the other hand, sorbitol accumulation decreases with the increase in fruit development and it is prominent sugar in unripe fruits. However, it is very minute in fully ripened fruits and its percentage is considerably lower as compared to the total sugar contents. There is also increase in fructose and glucose with maturation [57]. About 90% sugar accumulation occurs first 14 days of fruit maturation. Sucrose, fructose and glucose are main sugars in ripe fruits while there is small proportion of sorbitol. Galactose is another important sugar present in loquat fruit but in very small quantity [41]. The quantity and type of sugar directly depends upon the climatic conditions and cultivar [11]. Observed the sugar profile of ‘Mogi’ and proposed that major sugars were glucose, fructose and sucrose. Similarly, major sugar in ‘Mogi’ is fructose that ranges from 3.7–4.0 mg per 100 g FW; while, sucrose is most prominent in ‘Tanaka’ [39]. The sugar profile evaluation of 12 loquat cultivars exhibited that type of sugar depends upon the flesh color of fruit [5]. In white– fleshed cultivars, the amount of sucrose was significantly higher than that of orange–fleshed ones. Sucrose declines rapidly during storage; however, there is negligible decline in fructose and glucose in similar conditions [11]. This decrease in sucrose in associated with the activities of sucrose metabolizing enzymes [58].
Kanayama divided sugar metabolism into hexose, sorbitol and sucrose metabolism on the basis of sugar production in ripening loquat fruits [59]. Sucrose is mainly metabolized by Acid Invertase (AI), Sucrose Synthase (SS) and Sucrose Phosphate Synthase (SPS) enzymes while Sorbitol Oxidase (SOX), Sorbitol Dehydrogenase (SDH) and sorbitol–6–Phosphate Dehydrogenase (S6PDH) are chief enzymes for sorbitol metabolism in ripening fruits [60]. The role of these enzymes in ripening fruit is very significant as these convert reducing sugars into non–reducing sugars that are the constituent of ripe loquat fruits. Higher AI, SPS, SDH and SS enzymes activities were observed in ‘Mogi’ fruits with higher sugar accumulation at ripening stage although there were low activities of these enzymes at early developmental stages [61]. There is direct correlation between sugar metabolism and the activities of these enzymes throughout fruit development that leads to higher non–reducing sugars at mature stage. During fruit development, there was increased S6PDH transcription level with higher activity and protein synthesis that was subsequently reduced in relation to NAD+–SDH which clearly exhibit that there may be sorbitol accumulation in fruits [62].
A comparative analysis of ‘Bahong’, ‘Qingzhong’ and ‘Jidanbai’ loquats revealed that SPP and SS are chief enzymes in sugar metabolism; hence there is continuous alteration in SPS and SS enzymes as fruit development proceeds. There was higher sucrose accumulation in fruits with higher level of both these enzymes which indicated a positive association between sucrose and enzymes [63]. It was further investigated that there was significantly lower SDH, SS and AI enzymes during early stages of fruit development in ‘Dahongpao’ and ‘Ninghaibai’ that were consistently increased as ripening was advanced but comparatively white fleshed ‘Ninghaibai’ loquat maintained higher SS and AI enzymes than yellow fleshed ‘Dahongpao’ at ripening stage [64]. Transcriptional pattern of sugar regulating genes in loquat has not been extensively studied and there is no such report that clearly exhibit sugar accumulation with respect to gene expression. There is increasing concern to identify and clone the genes whose expression is responsible for sugar metabolism in loquat fruit. Successful cloning of fructokinase, sorbitol transporter protein, AI, SPS, SDH and SS encoding genes has been done.
Phenolic contents
Phenolics are very crucial secondary metabolites that are responsible for abiotic stress tolerance, seed dormancy, fruit color formation, antioxidative capacity and disease defense mechanism in plants. There is a significant change in the phenolic contents during maturation and ripening of loquat fruit [65]. Loquat fruit has high profile of phenolic contents and currently lignin, flavones, flavanone, flavonol, hydroxycinnamic and hydroxybenzoic acids have been identified although there is a huge variation of phenolics in different loquat fruit tissues with highest in seeds and minimum in flesh tissues [7]. The composition of different phenolic contents during fruit development in ‘Tanaka’, ‘Yukawa’, ‘Toi’, ‘Tsukumo’, Shiromogi’, ‘and Nagasakiwase ’and‘Mogi’ loquat exhibited huge variation at different fruit development stages [66]. They identified p–coumaric acid, ferulic acid, o–coumaric acid, epicatechin, 4–caffeoylquinic acid, protocatechuic acid, 5–p– feruloylquinic acid, hydroxybenzoic acid, neochlorogenic acid and 5–caffeoylquinic acid as major phenolics during fruit development. There was higher phenolic profile in young fruits which consistently declined throughout the maturation period with highest value of neochlorogenic acid at early stages while peak value in ripe fruit was shown by 5–caffeoylquinic acid, thereby exhibited that higher 5–caffeoylquinic acid is the maturity index of loquat fruit ripening. There was close association between phenolic contents and activities of hydroxycinnamoyl Coa: Quinate Hydroxycinnamoyl Transferase (CQT), 4–coumarate:Coa Ligase (4CL) and Phenyl Ammonia Lyase (PAL) that were high immediately after fruit set but there was decrease in their activities 3 weeks before harvest that dramatically rose at the time of harvest, clearly indicated the correlation between 5–caffeoylquinic acid and these enzymes. Moreover, it was also proposed that L–phenylalanine is the precursor for biosynthesis of cholorogenic acid. They also presented the major phenolic contents and their value at the time of maturity (Table 4). There is sharp increase in PPO enzyme activity at the time of maturation in ‘Mogi’ loquat that was reduced sharply at the ripening stage so phenolic contents decreased at the time of maturation and increased at ripening stage [11].
| Variety | Total phenolics | 5-caffeoylquinic acid | 3-caffeoylquinic acid | 4-caffeoylquinic acid | 5-feruloylquinic acid | hydroxybenzoic acid |
|---|---|---|---|---|---|---|
| ‘Yokohawa’ | 173.8 ± 12.3 | 90.70 ± 6.5 | 20.70 ± 3.1 | 2.15 ± 0.21 | 14.52 ± 2.5 | 7.82 ± 0.8 |
| ‘Toi’ | 158.3 ± 25.2 | 76.05 ± 8.2 | 19.21 ± 2.8 | 3.00 ± 0.24 | 8.51 ± 1.8 | 8.15 ± 0.7 |
| ‘Tsukumo’ | 110.0 ± 9.8 | 52.13 ± 5.8 | 16.60 ± 3.5 | 4.33 ± 0.34 | 2.81 ± 2.1 | 3.64 ± 0.5 |
| ‘Tanaka’ | 103.4 ± 11.2 | 55.82 ± 3.5 | 9.55 ± 3.5 | 0.81 ± 0.13 | 5.42 ± 1.7 | 2.65± 0.4 |
| ‘Shiromogi’ | 93.9 ± 8.6 | 33.11 ± 3.2 | 15.87 ± 3.1 | 1.14 ± 0.12 | 9.25 ± 1.5 | 4.08 ± 0.5 |
| ‘Mogi’ | 91.1 ± 7.9 | 42.54 ± 3.5 | 14.30 ± 2.4 | 2.00 ± 0.15 | 7.01 ± 1.3 | 4.01 ± 0.4 |
| ‘Nagasakiwase | 81.8 ± 8.3 | 32.92 ± 4.2 | 10.76 ± 2.2 | 0.52±0.08 | 4.47±0.7 | 2.24±0.3 |
Table 4: Phenolic compounds (milligrams per 100 g of fresh weight) in different varieties of loquat fruit at ripe stage.
Similarly, value of total phenolic contents varied from 253 to 140 μg g−1 FW in seven Turkish loquat cultivars, ‘Akko III’, ‘Hafif Cukurgobek’, ‘Sayda’, ‘KKTC’, ‘KKTC 4’, ‘Guzelyurt 6’ and ‘Champagne de Grasse’ [67]. The comparison of phenolic contents of six Chinese loquat cultivars including ‘Daguotaipingbai’, ‘Ninghaibai’, ‘Taxiahong’, ‘Taxiahuang’, ‘Taxiabai’ and ‘Taipingbai’ was observed by HPLC and it was proposed that o–coumaric acid, ellagic acid, ferulic acid, caffeic acid, 4–hydroxybenzoic acid, protocatechuic acid, 4–O– caffeoylquinic acid, neochlorogenic acid and chlorogenic acid were the main phenolic components in mature fruits; however, maximum phenolic content was exhibited in ‘Taxiahong’ loquat at maturation while minimum was shown in ‘Taipingbai’ [68-69]. Similarly, identified 11 different phenolic compounds including kaempferol–3–O–glucoside, kaempferol–3–O– rhamnoside, kaempferol–3–O–galactoside, quercetin–3–O– rhamnoside, quercetin–3–O–glucoside, quercetin–3–O– galactoside, 5–feruloylquinic acid, 3–caffeoylquinic acid, 4– caffeoylquinic acid, 5–caffeoylquinic acid and 3–p– coumaroylquinincacid in ‘Baozhu’, ‘Dahongpao’, ‘Dayeyangdun’, ‘Jiajiao’, ‘Luoyangqing’, ‘Ninghaibai’ and ‘Ruantiaobaisha’ in pulp and peel tissues of loquat cultivars with 5–caffeoylquinic acid as major constituent that accounts about 75% of total phenolic content in all cultivars.
Organic acid metabolism and fruit firmness
Production of acids is also an important indicator for the optimum fruit quality. In commercial loquat production, high acidity lowers the fruit quality. There are many organic acids in unripe loquat fruit including fumaric, succinic, citric and malic acid that contributes about 90% of total acids [44]. There is a continuous decline in organic acid contents in loquat fruit due to its utilization for respiratory metabolite energy and source of sugar production that are major constituent of sweetness in fruits. There are contradictions in the scientific investigations of different researchers [58]. Proposed quinate and malate as major organic acids in developing loquat pulp. Moreover, they also found very less amounts of β–coumarate, cis–aconitase, ferulate, tartrate, oxaloacetate, fumarate, α–ketoglutarate and isocitrate. In another study [70]. Detected six different acids (fumarate, puruvate, oxalate, tartarate, lactate and malate) in ‘Zaozhong’ and ‘Jiefangzhong’. There is gradual decline in the fruit firmness with the increase in the development phase. There is high pectin and lignin deposition on the cell of fruits at immature stage. PAL enzyme is the key factor for the synthesis of lignin. However, as the development precedes this pectin and lignin contents are being metabolized due to reduction in PAL enzyme activity and causes the change in fruit firmness and color development [71].
Flavor and aroma volatiles
Organolaptic quality is directly associated with the TA and TSS of the fruits at maturity stage. The flavor of loquat fruit is main characteristic to determine the fruit quality and it is due to certain volatile compounds. TSS of loquat fruit is one of the most important indicators that directly influence the fruit flavor. It has been observed by Hamauzu [39]. That optimum TSS for best quality loquat is 12° Brix. Sucrose, glucose and fructose are major soluble sugars in ‘Mogi’ loquat fruit; while, sorbitol is the major alcoholic acid. TA is another indicator that affects the flavor and organolaptic characteristics of loquat fruit. Moreover, there is close association of flavor with sugar to acid ratio in ripe fruit [58]. Proposed quinate and malate as major organic acids in developing loquat pulp. Moreover, they also found very less amounts of β–coumarate, cis–aconitase, ferulate, tartrate, oxaloacetate, fumarate, α–ketoglutarate and isocitrate. Other major constituents of flavor in loquat are 3‐hydroxy‐2‐ butanone and phenyl ethyl alcohol; while, methyl cinnamate, ethyl acetate and β‐ionone [72]. Aroma volatiles are considered to be related with quality of loquat fruit [71]. Reported that there are 78 aroma compounds which contribute the flavor of fresh loquat. The most significant compound is phenylacetaldehyde; while, β–ionone, hexanoic acid and hexanal are also present.
Fruit maturity stage can be determined by different physiological changes in loquat fruit. Titratable Acidity (TA), Total Soluble Solids (TSS), flesh firmness and TSS/TA ratio are the main indicators that can be used to assess the harvest maturity of loquat [73]. Organoleptic quality is directly associated with the TA and TSS of the fruits at maturity stage. Harvest maturity of loquat can be identified on the basis of aroma volatiles. Reported that there are 78 aroma compounds which contribute the flavor of fresh loquat. The most significant compound was phenylacetaldehyde; while, β–ionone, hexanoic acid and hexanal were also present. Sugars are the integral part of fruit flavor and quality. Sucrose is the predominant among asll other sugars in ripe loquat and accumulates at faster rate at the beginning of maturation. On the other hand, sorbitol degradation increases with the increase in fruit development and it is prominent sugar in unripe fruits. However, it is very minute in fully ripened fruits and its percentage is considerably lower as compared to the total sugar contents. There is also increase in fructose and glucose contents with maturation [57-58]. Demonstrated that level of carbohydrates is also potent parameter for fruit maturity. At maturation, there was higher glucose and fructose accumulation in loquat; however, sorbitol and sucrose were very low in yellow–fleshed loquat fruits. According to Kader the optimum TSS for the maturity of loquat is 10°Brix and often referred as the maturity indicator [73]. Reported that TSS/TA ratio should also be taken into account for the maturity assessment of loquat; however, fruit skin color can also be used for maturity of loquat. Harvest stage is directly correlated with the quality of loquat during postharvest storage and transportation. High degree of ripening at harvest stage ensures the maximum and optimum fruit quality for the consumers [74].
Loquat fruit falls in the category of minor fruits and it has only been studied on the basis of biochemical changes during fruit development. The extensive studies on preharvest physiology of loquat like other main fruits including citrus, mango, apple, grapes and date palm has not been conducted. Only a very few studies have been done to investigate molecular changes during fruit ripening. Moreover, there is little knowledge about harvesting techniques and field operations. There is no comprehensive study on genetic basis and proteomics of fruit ripening in relation to biochemical changes in loquat. Additionally, there is still a gap to explore the preharvest practices and their influence on postharvest storage capacity of loquat fruit.
[Crossref] [Google Scholar] [Indexed]
[Crossref] [Google Scholar] [Indexed]
[Crossref] [Google Scholar] [Indexed]
[Crossref] [Google Scholar] [Indexed]
[Crossref] [Google Scholar] [Indexed]
[Crossref] [Google Scholar] [Indexed]
[Crossref] [Google Scholar] [Indexed]
[Crossref] [Google Scholar] [Indexed]
[Crossref] [Google Scholar] [Indexed]
[Crossref] [Google Scholar] [Indexed]
[Crossref] [Google Scholar] [Indexed]
[Crossref] [Google Scholar] [Indexed]
[Crossref] [Google Scholar] [Indexed]
[Crossref] [Google Scholar] [Indexed]
[Crossref] [Google Scholar] [Indexed]
[Crossref] [Google Scholar] [Indexed]
[Crossref] [Google Scholar] [Indexed]
[Crossref] [Google Scholar] [Indexed]
[Crossref] [Google Scholar] [Indexed]
[Crossref] [Google Scholar] [Indexed]
[Crossref] [Google Scholar] [Indexed]
[Crossref]
[Crossref] [Google Scholar] [Indexed]
[Crossref] [Google Scholar] [Indexed]
[Crossref] [Google Scholar] [Indexed]
[Crossref] [Google Scholar] [Indexed]
[Crossref] [Google Scholar] [Indexed]
[Crossref] [Google Scholar] [Indexed]
[Crossref] [Google Scholar] [Indexed]
[Crossref] [Google Scholar] [Indexed]
[Crossref] [Google Scholar] [Indexed]
[Crossref] [Google Scholar] [Indexed]
[Crossref] [Google Scholar] [Indexed]
[Crossref] [Google Scholar] [Indexed]
[Crossref] [Google Scholar] [Indexed]
[Crossref] [Google Scholar] [Indexed]
[Crossref] [Google Scholar] [Indexed]
[Crossref] [Google Scholar] [Indexed]
[Crossref] [Google Scholar] [Indexed]
Citation: Shoaib Shah HM, Waheedb A, Buttc MA (2022) Preharvest Technology and Dynamics of Loquat Fruit Ripening. J Hortic. 9:306
Received: 07-Feb-2022, Manuscript No. HORTICULTURE-22-15415; Editor assigned: 10-Feb-2022, Pre QC No. HORTICULTURE-22-15415(QC); Reviewed: 24-Feb-2022, QC No. HORTICULTURE-22-15415; Revised: 07-Apr-2022, Manuscript No. HORTICULTURE-22-15415(R); Published: 15-Apr-2022
Copyright: © 2022 Shoaib Shah HM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.